Hydrate formation temperature prediction
•
2 likes•638 views
Hydrate formation is a well-known risk in natural gas wells and pipelines and is managed by temperature control or the injection of hydrate inhibitors, most commonly methanol, which lower the hydrate formation temperature to below the minimum expected operating temperature.
Report
Share
Report
Share
Download to read offline
Recommended
Thermodynamic Property Relations
Properties of pure substances
Maxwell Relations
P-v diagram of a pure substance
pvt surface diagram
How to read steam table using an exampleThermodynamic property relations IP University Thermal science

Thermodynamic property relations IP University Thermal scienceANKIT SAXENA Asst. Prof. @ Dr. Akhilesh Das Gupta Institute of Technology and Management, New Delhi
Recommended
Thermodynamic Property Relations
Properties of pure substances
Maxwell Relations
P-v diagram of a pure substance
pvt surface diagram
How to read steam table using an exampleThermodynamic property relations IP University Thermal science

Thermodynamic property relations IP University Thermal scienceANKIT SAXENA Asst. Prof. @ Dr. Akhilesh Das Gupta Institute of Technology and Management, New Delhi
More Related Content
What's hot
What's hot (20)
Thermodynamics lab experiment 01_ bolyes law_part_1_expansion

Thermodynamics lab experiment 01_ bolyes law_part_1_expansion
Thermodynamics lab experiment 02_bolyes law_part_2_compression

Thermodynamics lab experiment 02_bolyes law_part_2_compression
ME6301 ENGINEERING THERMODYNAMICS SHORT QUESTIONS AND ANSWERS - UNIT IV

ME6301 ENGINEERING THERMODYNAMICS SHORT QUESTIONS AND ANSWERS - UNIT IV
Similar to Hydrate formation temperature prediction
Similar to Hydrate formation temperature prediction (20)
Analysis of the Thermal Efficiency of Condensing Wall-Hung Boiler

Analysis of the Thermal Efficiency of Condensing Wall-Hung Boiler
Combined-Avogadros-and-Ideal-Gas-Laws [Autosaved].pptx![Combined-Avogadros-and-Ideal-Gas-Laws [Autosaved].pptx](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Combined-Avogadros-and-Ideal-Gas-Laws [Autosaved].pptx](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Combined-Avogadros-and-Ideal-Gas-Laws [Autosaved].pptx
ch13_part1 (Reacting mixtures and combustion, Heating values, Gibbs function)...

ch13_part1 (Reacting mixtures and combustion, Heating values, Gibbs function)...
Evaluating Properties For mechanical and Industrial Engineering

Evaluating Properties For mechanical and Industrial Engineering
Thermodynamyc Enginering. Chapter 8: PRODUCTION OF POWER FROM HEAT

Thermodynamyc Enginering. Chapter 8: PRODUCTION OF POWER FROM HEAT
CFD Simulation Studies for Vertical Temperature Profile,.pdf

CFD Simulation Studies for Vertical Temperature Profile,.pdf
More from Mohamed A. Hassan, CEng MIChemE
More from Mohamed A. Hassan, CEng MIChemE (14)
Recently uploaded
Recently uploaded (20)
PE 459 LECTURE 2- natural gas basic concepts and properties

PE 459 LECTURE 2- natural gas basic concepts and properties
Digital Communication Essentials: DPCM, DM, and ADM .pptx

Digital Communication Essentials: DPCM, DM, and ADM .pptx
Passive Air Cooling System and Solar Water Heater.ppt

Passive Air Cooling System and Solar Water Heater.ppt
Max. shear stress theory-Maximum Shear Stress Theory Maximum Distortional ...

Max. shear stress theory-Maximum Shear Stress Theory Maximum Distortional ...
scipt v1.pptxcxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx...

scipt v1.pptxcxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx...
Convergence of Robotics and Gen AI offers excellent opportunities for Entrepr...

Convergence of Robotics and Gen AI offers excellent opportunities for Entrepr...
Compressing and Sparsifying LLM in GenAI Applications

Compressing and Sparsifying LLM in GenAI Applications
NO1 Top No1 Amil Baba In Azad Kashmir, Kashmir Black Magic Specialist Expert ...

NO1 Top No1 Amil Baba In Azad Kashmir, Kashmir Black Magic Specialist Expert ...
1_Introduction + EAM Vocabulary + how to navigate in EAM.pdf

1_Introduction + EAM Vocabulary + how to navigate in EAM.pdf
Hydrate formation temperature prediction
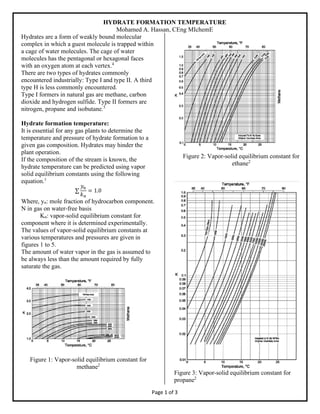
- 1. HYDRATE FORMATION TEMPERATURE Mohamed A. Hassan, CEng MIchemE Page 1 of 3 Hydrates are a form of weakly bound molecular complex in which a guest molecule is trapped within a cage of water molecules. The cage of water molecules has the pentagonal or hexagonal faces with an oxygen atom at each vertex.4 There are two types of hydrates commonly encountered industrially: Type I and type II. A third type H is less commonly encountered. Type I formers in natural gas are methane, carbon dioxide and hydrogen sulfide. Type II formers are nitrogen, propane and isobutane.5 Hydrate formation temperature: It is essential for any gas plants to determine the temperature and pressure of hydrate formation to a given gas composition. Hydrates may hinder the plant operation. If the composition of the stream is known, the hydrate temperature can be predicted using vapor solid equilibrium constants using the following equation.1 ∑ 𝑦𝑛 𝑘 𝑛 = 1.0 Where, yn: mole fraction of hydrocarbon component. N in gas on water-free basis Kn: vapor-solid equilibrium constant for component where it is determined experimentally. The values of vapor-solid equilibrium constants at various temperatures and pressures are given in figures 1 to 5. The amount of water vapor in the gas is assumed to be always less than the amount required by fully saturate the gas. Figure 1: Vapor-solid equilibrium constant for methane2 Figure 2: Vapor-solid equilibrium constant for ethane2 Figure 3: Vapor-solid equilibrium constant for propane2
- 2. HYDRATE FORMATION TEMPERATURE Mohamed A. Hassan, CEng MIchemE Page 2 of 3 Figure 4: vapor-solid equilibrium constant for isobutane2 Figure 5: Vapor-solid equilibrium constant for CO2 & H2S2 For components heavier than butane, the equilibrium constant is taken as infinity.1 Figure 6: Vapor-solid equilibrium constant for n- butane2 • The steps for determining the hydrate temperature at a given system pressure as as follows: 1. Assume a hydrate formation temperature 2. Determine Kn for each component 3. Calculate (yn/kn) for each component 4. Sum the values of (yn/kn). 5. Repeat steps until the summation of (yn/kn) is equal to 1.0 Example: The following example is try handout calculations of hydrate formation temperature and compare the results with simulation softwares results. The gas is composed of 27.3 % methane, 58.7 % ethane, 10.8 % propane and 3.2 % butane.
- 3. HYDRATE FORMATION TEMPERATURE Mohamed A. Hassan, CEng MIchemE Page 3 of 3 Determine: 1. The hydrate formation temperature at 3500 KPa using the above hand-out calculations and simulation software 2. Draw gas hydrate curve using the handout calculations and simulation software. 1. Determine hydrate formation temperature: Comp. Y K10 Y/K10 K15 Y/k15 Methane 0.27 1.45 0.188 1.62 0.16 Ethane 0.58 0.34 1.72 0.95 0.617 C3 0.1 0.1 1.08 ∞ 0 C4 0.03 0.2 0.16 ∞ 0 1 3.15 0.78 By interpolation, the hydrate formation temperature is 14.535 C where summation of (Y/K) equals one. The Aspen Hysys hydrate formation temperature is 14.7927o C, while pro/II result is 14.73o C. 2. Drawing the hydrate curve: Repeating the same calculations at different pressures to draw hydrate curve, then using Pro/II and Aspen Hysys to find the hydrate temperature. Pressure (KPa) Model T Pro/II T HYSYS T 3500 14.535 14.73 14.7927 3000 13.945 13.44 13.5001 2500 11.511 11.88 11.9643 2000 10.004 10.09 10.1664 1500 7.385 7.69 7.755 Figure 7: hydrate curve On the left side of the curve is the hydrate formation region. When pressure and temperature are in this region, water and gas will start to form hydrate. On the right side of the curve is the non-hydrate formation region. When pressure and temperature are in this region, water and gas will not form hydrate. The hydrate formation temperature can be predicted using the specific gravity and pressure using the figure, but this method is being used when gas composition is not known. Figure 8: pressure-temperature curve for predicting hydrate formation temperature2 References: 1. Surface Production Operations, 2nd edition, Volume 2, Design of gas handling systems and facilities, Maurice Stewart. 2. Gas processors suppliers association GPSA Engineering data book, 10th edition. 3. Gas dehydration field manual, Maurice Stewart. 4. Carbon capture and storage, Stephan A. Rackley. 5. Natural gas hydrates – A guide for engineers, 3rd edition, 2014. 1400 1900 2400 2900 3400 3900 7 9 11 13 15 PressurekPa Temperature C Model Pro/II HYSYS
