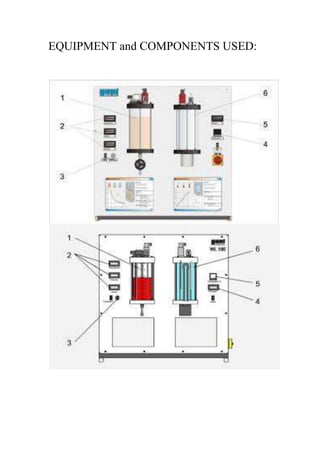
The document describes an experiment conducted at Koya University to demonstrate Boyle's Law, which states that pressure is inversely proportional to volume for a gas at constant temperature. The experiment aims to quantitatively show this relationship using a gas sample in a closed hypodermic syringe. Boyle's Law, first stated in 1662, is applicable in various practical applications, such as in internal-combustion engines.