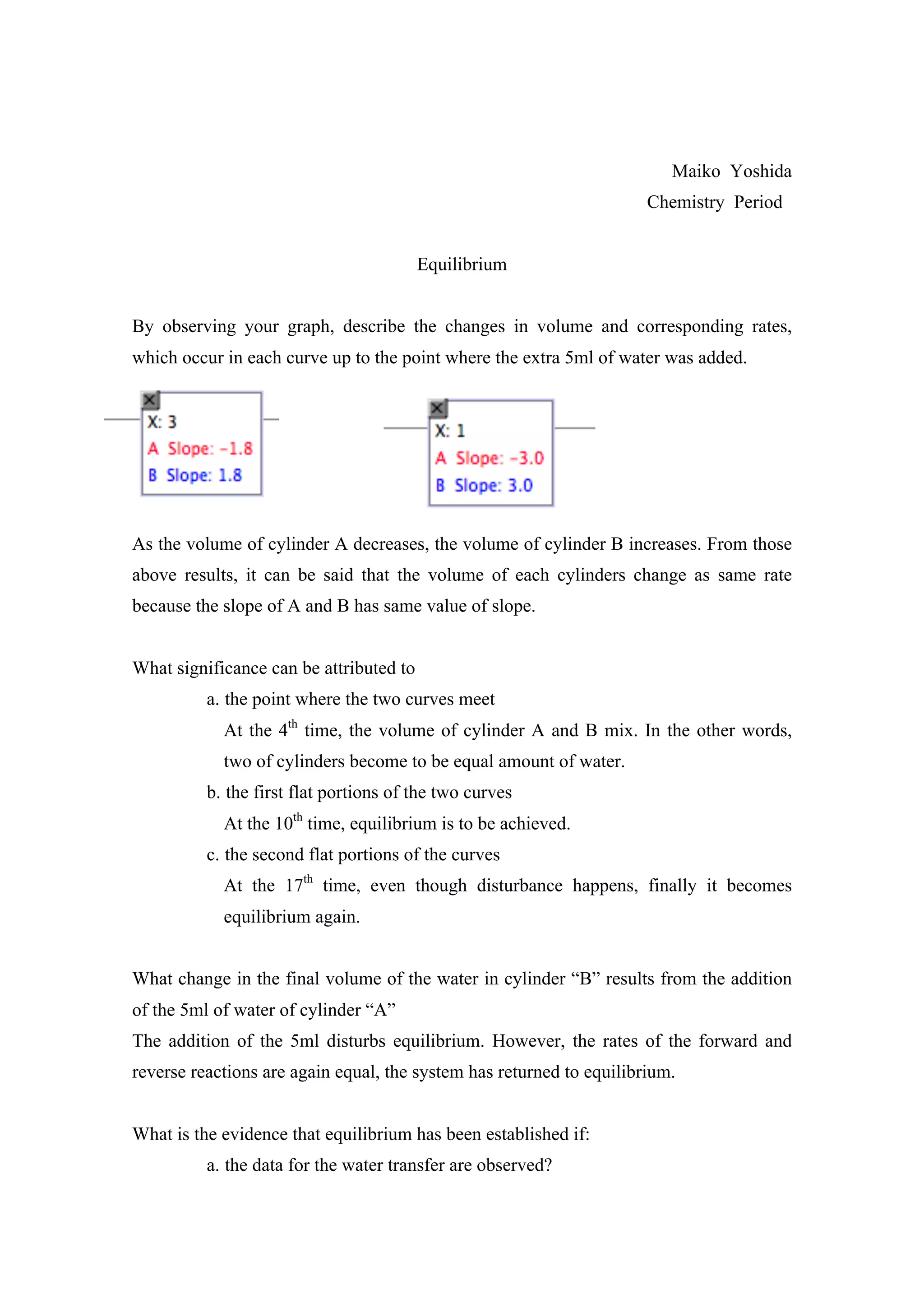
This document describes an experiment observing the equilibrium of water transferring between two cylinders. It notes that at the point where the volumes of each cylinder are equal, the system has reached equilibrium. It also discusses how adding 5ml of water disturbs the equilibrium but the system returns to equilibrium again. The evidence that equilibrium is established is the identical, flat data observed over time in the plotted graph. This system is considered "closed" as it cannot exchange matter or energy with its surroundings.