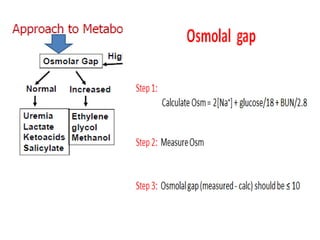
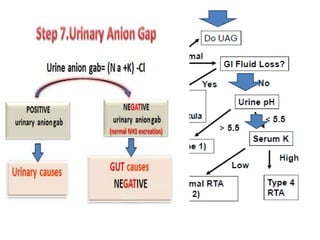
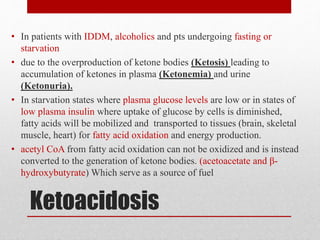
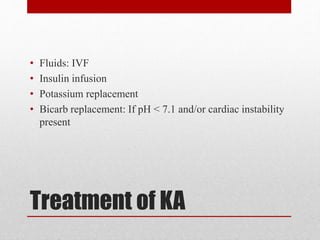
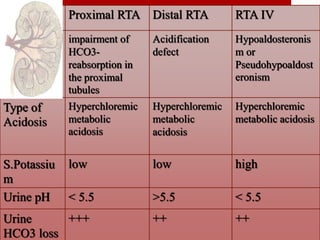
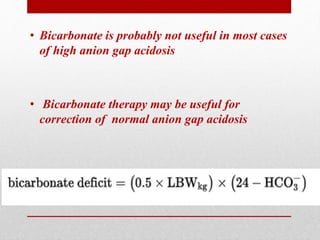
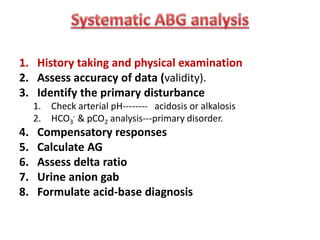
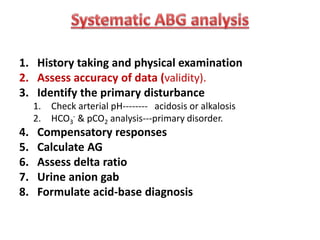
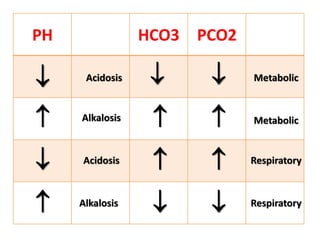
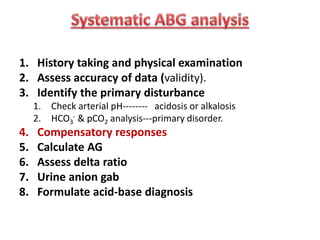
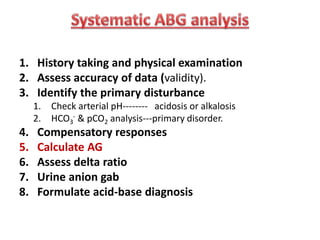
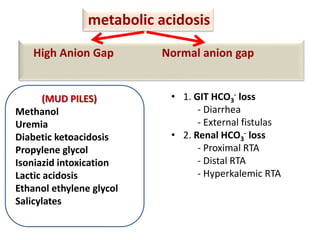
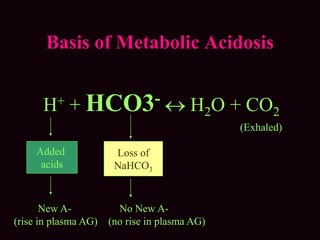
This document outlines an 8-step approach to assessing metabolic acidosis: 1) take history and do exam, 2) assess data validity, 3) identify primary disturbance via pH, HCO3, and pCO2 analysis, 4) examine compensatory responses, 5) calculate anion gap, 6) assess delta ratio, 7) check urine anions, 8) make an acid-base diagnosis. Key causes of metabolic acidosis include ketoacidosis, lactic acidosis, and renal tubular acidosis resulting from bicarbonate loss. Treatment depends on the underlying cause but may include fluid resuscitation, insulin, bicarbonate supplementation, and addressing precipitating factors.

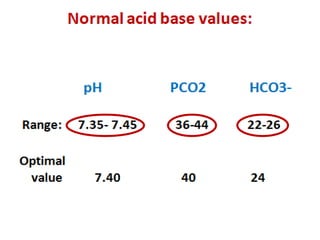


![Metabolic acidosis
Expected pCO2 = 1.5 x [HCO3] + 8 (range: +/- 2)
Metabolic alkalosis
Expected pCO2 = 0.7 [HCO3] + 20 (range: +/- 5)
“If the actual pCO2 or [HCO3
-]
is different from the predicted values,
You must suspect a 2nd acid-base disorder”](https://image.slidesharecdn.com/m-170130151433/85/Metabolic-acidosis-ABG-11-320.jpg)

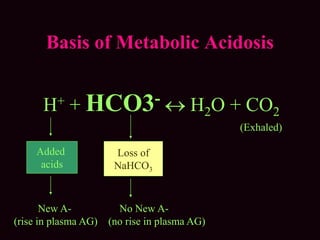
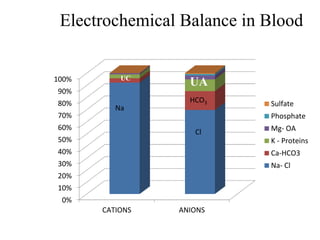
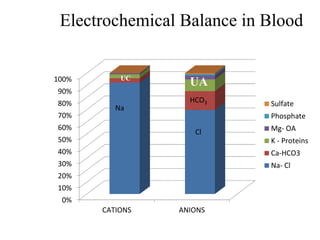
![• (Na + K) + UC = (Cl + HCO3) + UA
• The anion gap is defined as the quantity of
anions not balanced by cations.
• Anion Gap= measured cation- measured
anion.
• AG = [Na + K] – (Cl + HCO3) = 12 ± 4 meq/L
• Corrected AG (in Hypoalbuminemia):
4-alb*2.5](https://image.slidesharecdn.com/m-170130151433/85/Metabolic-acidosis-ABG-16-320.jpg)
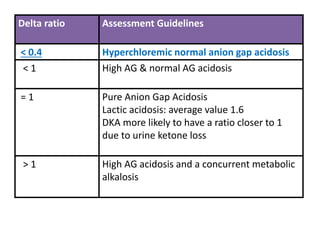
![Delta ratio= ∆ Anion gap/∆ [HCO3-]
∆ Anion gap = (AG-12)
∆ [HCO3-] = (24 - [HCO3-])](https://image.slidesharecdn.com/m-170130151433/85/Metabolic-acidosis-ABG-21-320.jpg)