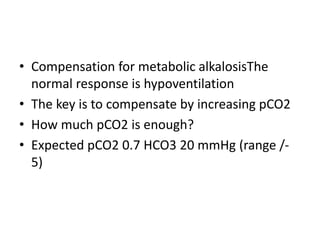
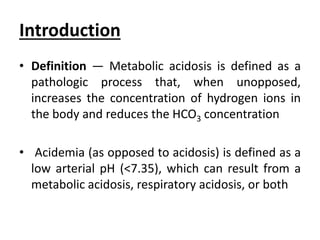
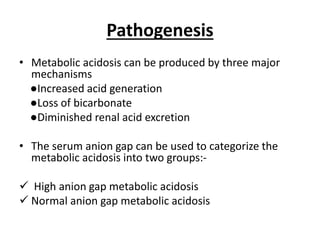
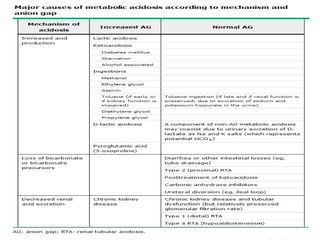
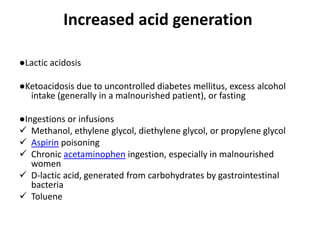
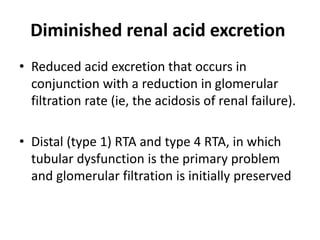
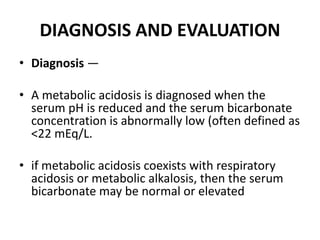
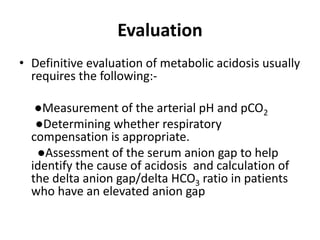
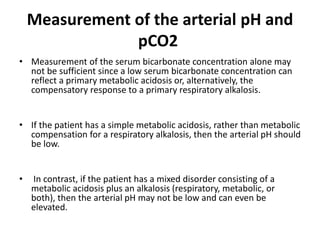
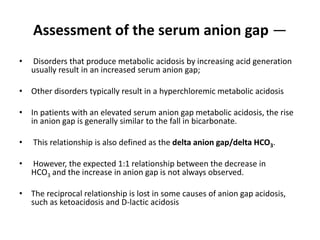
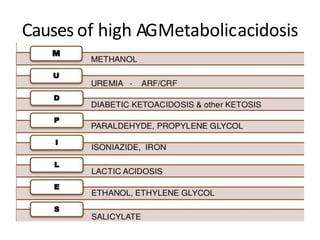
This document provides an overview of metabolic acidosis and alkalosis. It defines metabolic acidosis and acidemia, and describes the three main mechanisms that can produce metabolic acidosis: increased acid generation, loss of bicarbonate, and diminished renal acid excretion. Specific causes of metabolic acidosis like lactic acidosis and ketoacidosis are discussed. The document also covers metabolic alkalosis, describing mechanisms like intracellular shifts of hydrogen ions and gastrointestinal or renal losses of hydrogen ions. Diagnosis and treatment of both conditions in discussed.






![• The expected "normal" value for the anion gap must be adjusted
downward in patients with hypoalbuminemia. The serum anion gap
falls by 2.3 to 2.5 mEq/L for every 1 g/dL (10 g/L) reduction in the
serum albumin concentration.
• Corrected serum anion gap = (Serum anion gap measured) +
(2.5 x [4.5 - Observed serum albumin])
• In addition to hypoalbuminemia, marked hyperkalemia may affect
the interpretation of the anion gap. Potassium is an "unmeasured"
cation using the equation that does not include the serum K.
• Thus, a serum K of 6 mEq/L will reduce the anion gap by 2 mEq/L.
Hypercalcemia and/or hypermagnesemia will similarly reduce the
anion gap.](https://image.slidesharecdn.com/metabolicacidosis-200827155016/85/Metabolic-acidosis-16-320.jpg)



![Treatment
• Treatment of metabolic acidosis with alkali should be
reserved for severe acidemia except when the patient has
no “potential HCO3 −” in plasma.
The potential [HCO3 −] can be estimated from the increment
(Δ) in the AG (ΔAG = patient’s AG – 10), only if the acid
anion that has accumulated in plasma is metabolizable
(i.e., β-hydroxybutyrate, acetoacetate, and lactate).
Conversely nonmetabolizable anions that may accumulate in
advanced stage CKD or after toxin ingestion are not
metabolizable and do not represent “potential” HCO3−](https://image.slidesharecdn.com/metabolicacidosis-200827155016/85/Metabolic-acidosis-22-320.jpg)
![• With acute CKD improvement in kidney function to
replenish the [HCO3 −] deficit is a slow and often
unpredictable process.
• Consequently, patients with a normal AG acidosis
(hyperchloremic acidosis) or an AG attributable to a
nonmetabolizable anion due to advanced kidney failure
should receive alkali therapy, either PO (NaHCO3 or Shohl’s
solution) or IV (NaHCO3), in an amount necessary to slowly
increase the plasma [HCO3 −] to a target value of 22
mmol/L.
• Nevertheless, overcorrection should be avoided.](https://image.slidesharecdn.com/metabolicacidosis-200827155016/85/Metabolic-acidosis-23-320.jpg)

![• Administration of alkali requires careful monitoring of
plasma electrolytes, especially the plasma [K+], during the
course of therapy.
• A reasonable initial goal is to increase the [HCO3 −] to 10–
12 mmol/L and the pH to ∼7.20, but clearly not to increase
these values to normal.
• Estimation of the “bicarbonate deficit” by calculation of the
volume of distribution of bicarbonate is often taught but is
unnecessary and may result in administration of excessive
amounts of alkali.](https://image.slidesharecdn.com/metabolicacidosis-200827155016/85/Metabolic-acidosis-25-320.jpg)