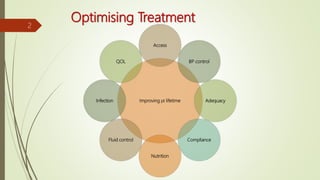
This document summarizes key aspects of fluid management in peritoneal dialysis (PD) patients. It discusses optimizing PD prescriptions to balance adequate solute clearance while avoiding excess dialysis fluid exposure. Factors like residual renal function, membrane characteristics, fill volume and dwell time are considered. Monitoring adequacy includes measuring clearances and adjusting therapy if targets are not met. Guidelines recommend strategies to preserve renal function like ACEi/ARB use and avoiding dehydration.