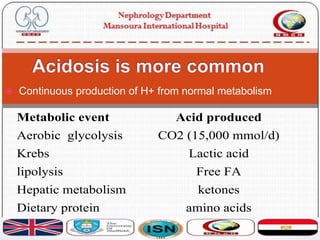
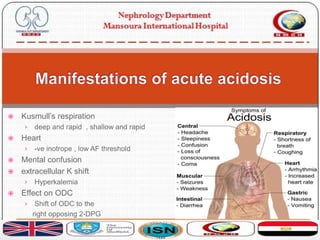
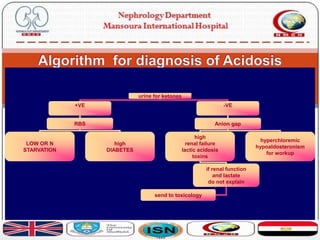
This document discusses acid-base disorders and their management. It provides an overview of acid-base physiology, the approach to evaluating acid-base disorders, common causes and types of acid-base disorders, and guidelines for correcting acid-base imbalances. Key points include: evaluating the primary disturbance, compensatory responses, and anion gap; causes of high vs normal vs low anion gap acidosis; hazards of over-correcting alkalosis; and only treating with bicarbonate if pH is below 7.2.




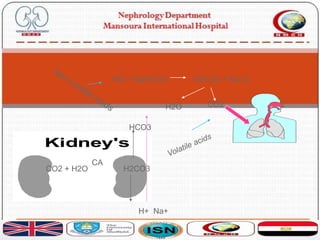
![ Acid-base balance is assessed in terms of CO2-HCO3
buffer system. It is expressed in pH:
pH = 6.10 + log ([HCO3-] ÷ [0.03 x PCO2])
Large number of metabolic events are sensitive to pH
mainly brain and heart
That is why a number of mechanisms are present in acid
base regulation holding blood pH in narrow limits
7.38-7.42](https://image.slidesharecdn.com/acidbasebalance-171004210543/85/Acid-base-balance-6-320.jpg)












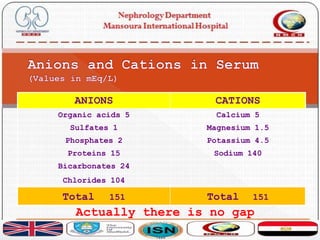
![ If you measure all the anions they should be equal to all the
cations
› cations - anions = zero
it is difficult to measure them all.
An easy way is measure the most abundant
[Na + K] - [HCO3 + Cl] = [144] - [129] = 16
Why is this difference? Are the cations more?
Of course not](https://image.slidesharecdn.com/acidbasebalance-171004210543/85/Acid-base-balance-24-320.jpg)
![ It is due to presence of unmeasured anions (and cations) and if you
measure them all definitely your sum will be zero
[Na + K + UC] - [HCO3 + Cl + UA] = 0
Unmeasured anions are mainly albumin and proteins
Unmeasured cations are mainly Ca and Mg
under normal conditions:
[Na + K] - [HCO3 + Cl] = 15 +/- 2
This is called normal anion gap](https://image.slidesharecdn.com/acidbasebalance-171004210543/85/Acid-base-balance-25-320.jpg)

![› There is a load of non chloride containing acid e.g. lactic
acid
› lactic acid + NaHCO3 Na lactate + H2CO3
› There is a fall in HCO3 conc and rise in lactate
› calc: [Na + K] - [HCO3 + Cl] = > 17
› The fall in HCO3 is compensated by the unmeasured
lactate](https://image.slidesharecdn.com/acidbasebalance-171004210543/85/Acid-base-balance-27-320.jpg)
![ There is addition of a Cl acid e.g. HCl or Loss of
HCO3 (with renal absorption of chloride)
› HCl + NaHCO3 n NaCl + H2CO3
› There is fall in HCO3 concentration and rise in chloride
› calc: [Na + K] - [HCO3 + Cl] = 15 +/- 2
since chloride is increased it is easier to name it
hyperchloremic acidosis](https://image.slidesharecdn.com/acidbasebalance-171004210543/85/Acid-base-balance-28-320.jpg)







![ Judge your decision by the pH and never by the HCO3
or CO2 levels
Do not give HCO3 unless pH is lower than 7.2 (7.1 by
some authors)
Calculate the deficit by the formula
[24-HCO3] x 50% of body weight
Correction should be slowly over 3 hours guided by
hourly blood gases and HCO3](https://image.slidesharecdn.com/acidbasebalance-171004210543/85/Acid-base-balance-38-320.jpg)
