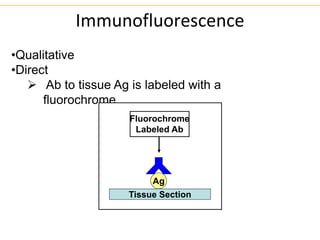
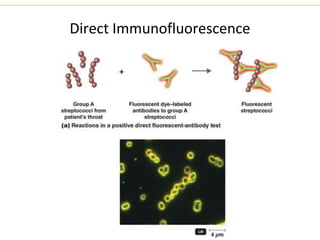
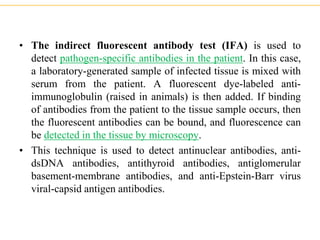
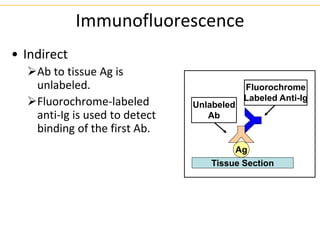
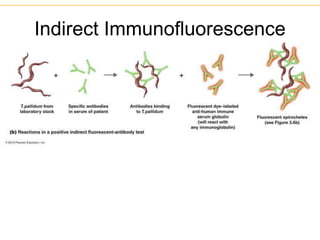
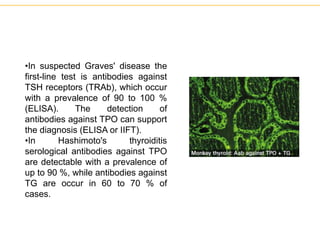
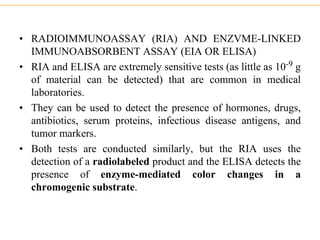
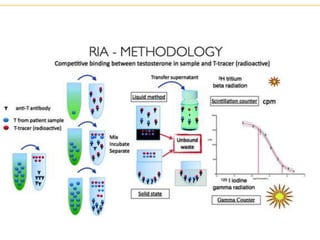
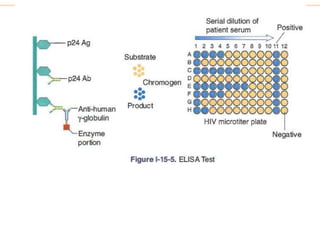
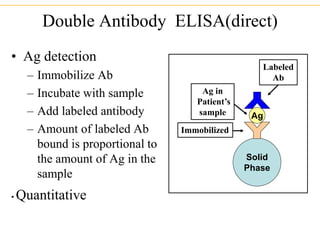
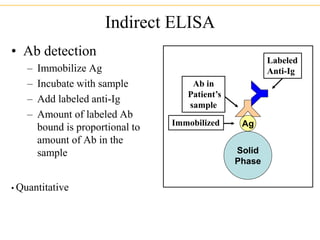
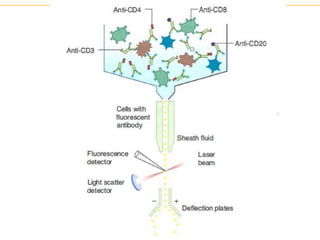
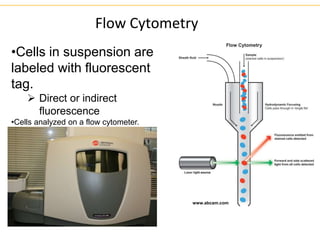
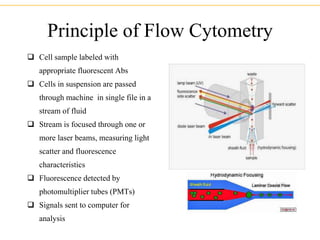
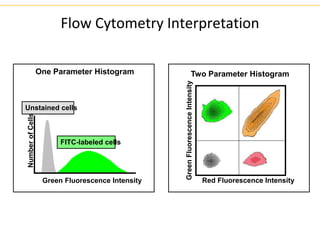
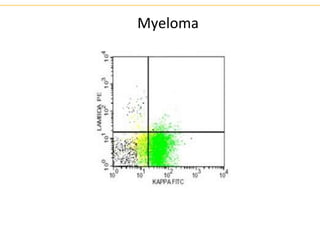
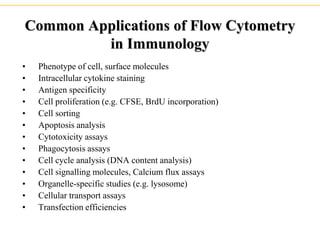
The document describes several laboratory techniques used in immunology, including antigen-antibody interactions, fluorescent antibody tests, radioimmunoassay/ELISA, and flow cytometry. Antigen-antibody interactions form the basis of precipitation and agglutination tests. Fluorescent antibody tests use fluorescent dyes to detect the presence of antigens or antibodies. Radioimmunoassay and ELISA are sensitive techniques to detect proteins and markers. Flow cytometry uses fluorescent antibodies to rapidly analyze cell populations based on surface marker binding.