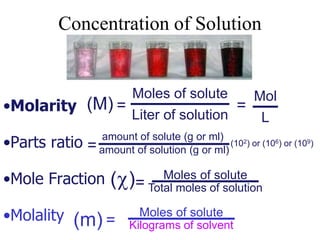
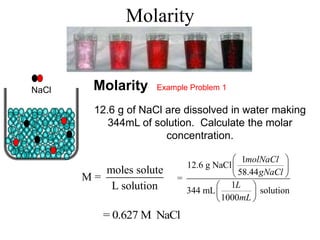
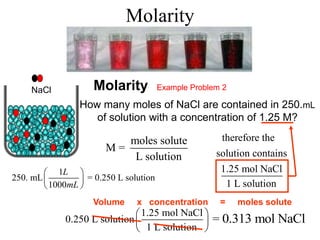
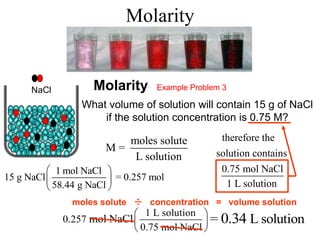
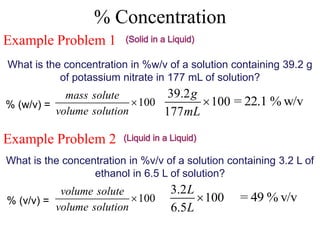
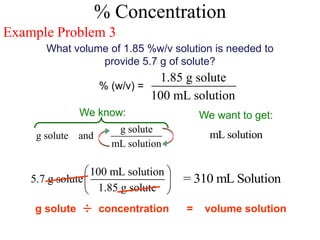
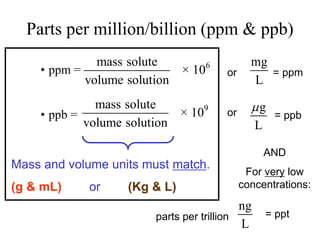
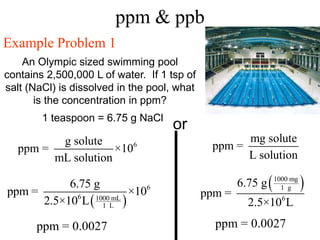
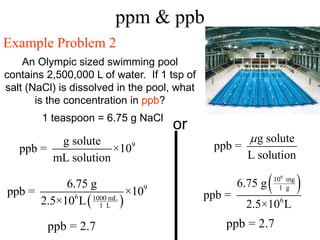
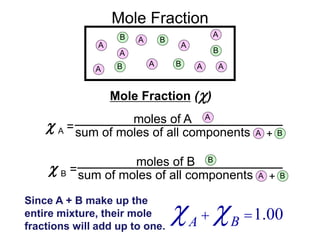
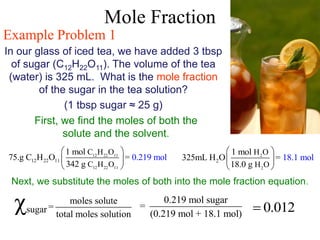
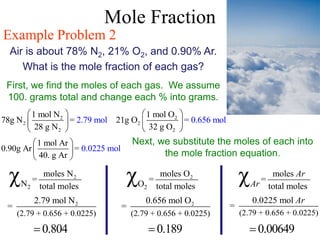
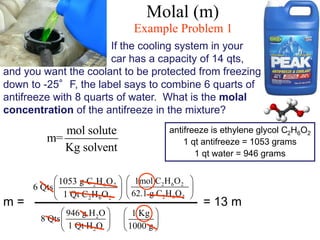
This document defines and provides examples of different types of concentration units used to quantify the amount of solute dissolved in a solution. It discusses molarity, percent concentration, parts per million/billion, mole fraction, and molality. Example problems are provided for each type of unit to demonstrate how to calculate concentration from given amounts of solute and solvent.