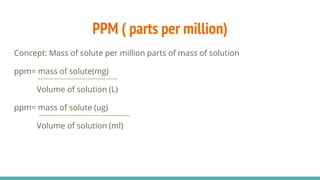
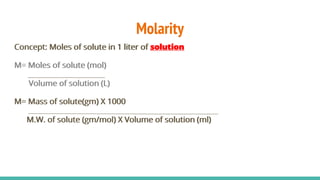
The document provides information about various concentration units used in chemistry such as molarity, molality, normality, formality, percentage solutions, parts per million (ppm), specific gravity, and dilution calculations. It defines each term, provides examples of calculations using the relevant formulas, and explains how to perform dilutions to make solutions with lower concentrations from more concentrated stock solutions. Key formulas covered include those for molarity (M= moles solute/L solution), molality (m= moles solute/kg solvent), and dilution (C1V1=C2V2).



![Find the molarity of solution prepared by dissolving 6.75 g of NaCl into 452 ml
of D.W. [ M.W. of NaCl = 58.4 g/mol ]
M= Mass of solute(gm) X 1000
M.W. of solute (gm/mol) X Volume of solution (ml)
M= 6.75 X 1000
58.4 X 452
=0.2557 M](https://image.slidesharecdn.com/solutions-210405045348/85/Solutions-5-320.jpg)
![How many grams of MgCl2 is required to make 500ml 0.5 M MgCl2? [ M.W. of
MgCl2 =95.21 gm/mol]
M= Mass of solute(gm) X 1000
M.W. of solute (gm/mol) X Volume of solution (ml)
0.5 = x X 1000
95.21 X 500
X = 0.5 X95.21 X 500
1000
=23.802 gm](https://image.slidesharecdn.com/solutions-210405045348/85/Solutions-6-320.jpg)
![What is molarity of 250 ml solution containing 0.35 moles of NaCl? [ M.W. of
NaCl = 58.4 g/mol ]
M= Moles of solute (mol)
Volume of solution (L)
= 0.35
250/1000
= 0.35
0.25
= 1.4 M](https://image.slidesharecdn.com/solutions-210405045348/85/Solutions-7-320.jpg)



![Calculate the normality of 1.80 g H2C2O4 dissolved in 150 ml of solution.
[M.W. =90 gm/mol]
0.267 N](https://image.slidesharecdn.com/solutions-210405045348/85/Solutions-11-320.jpg)