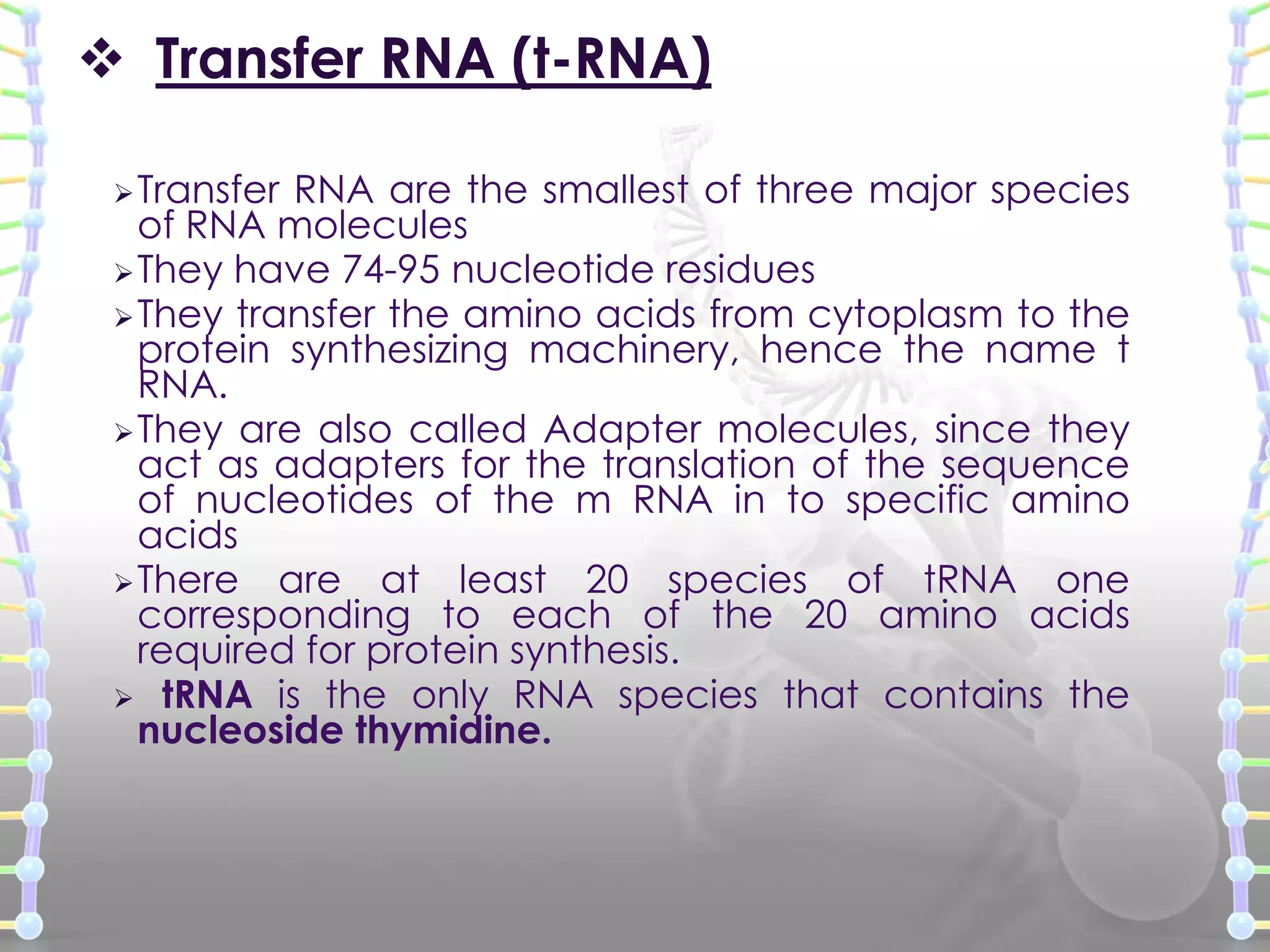
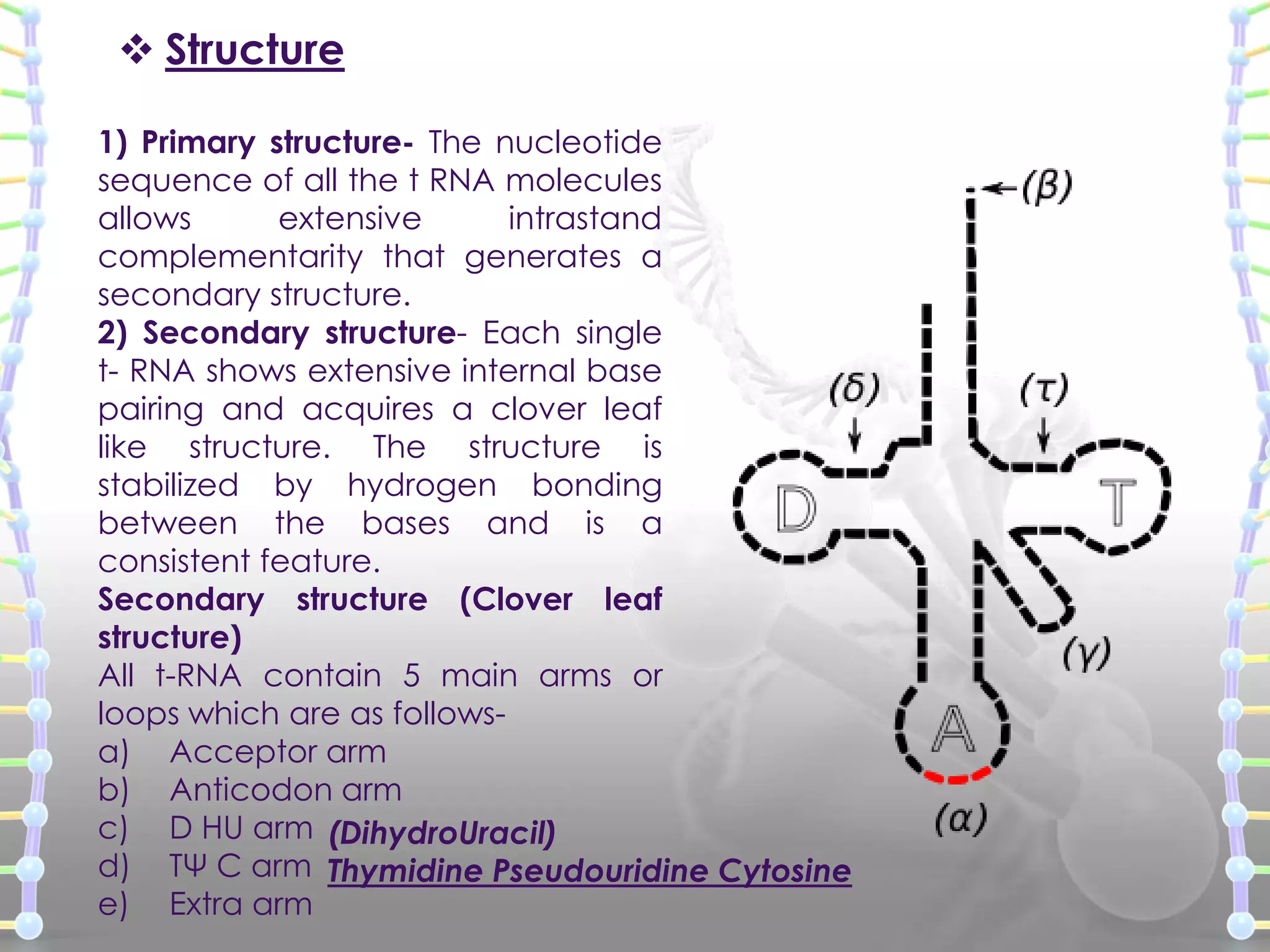
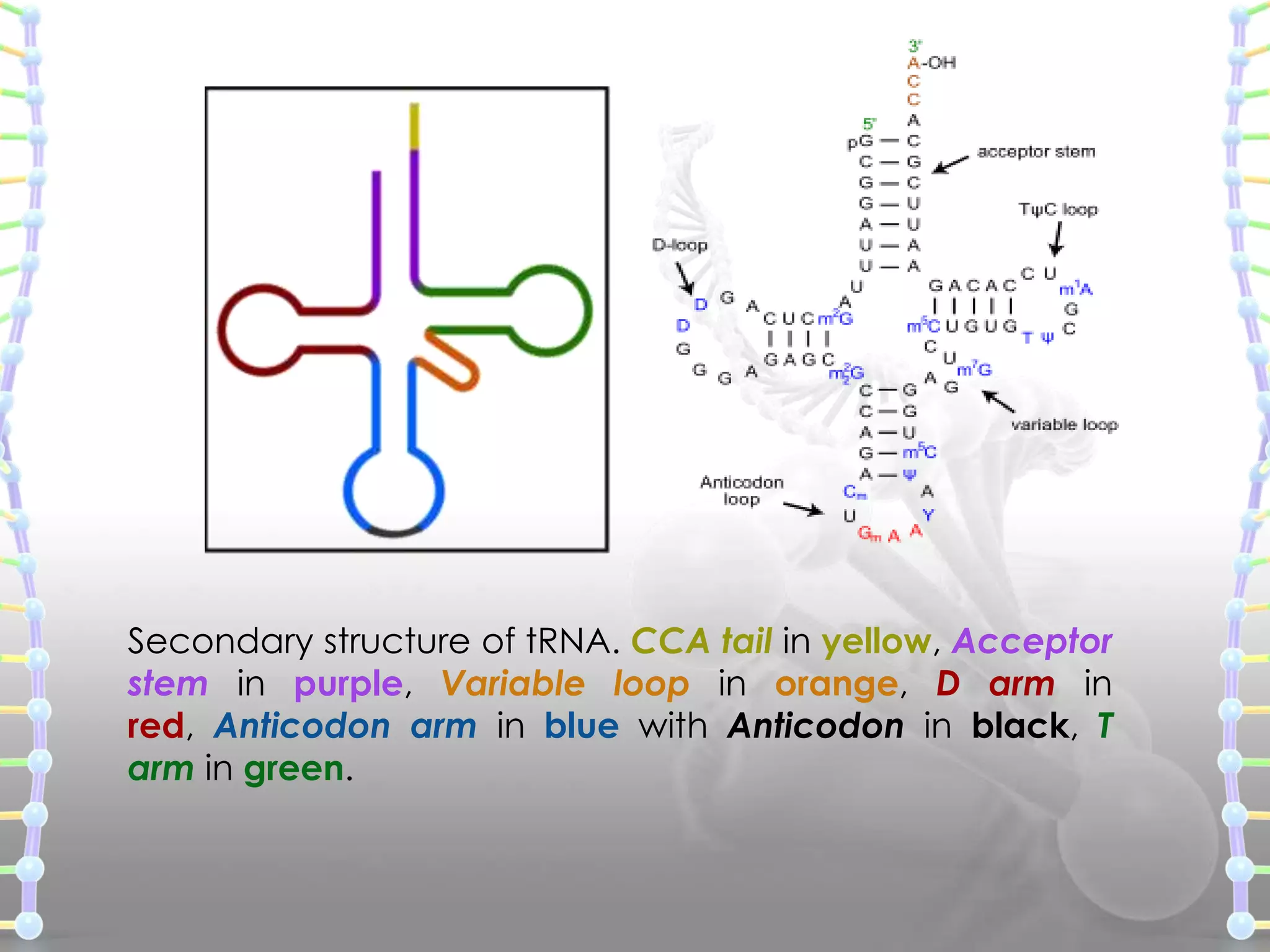
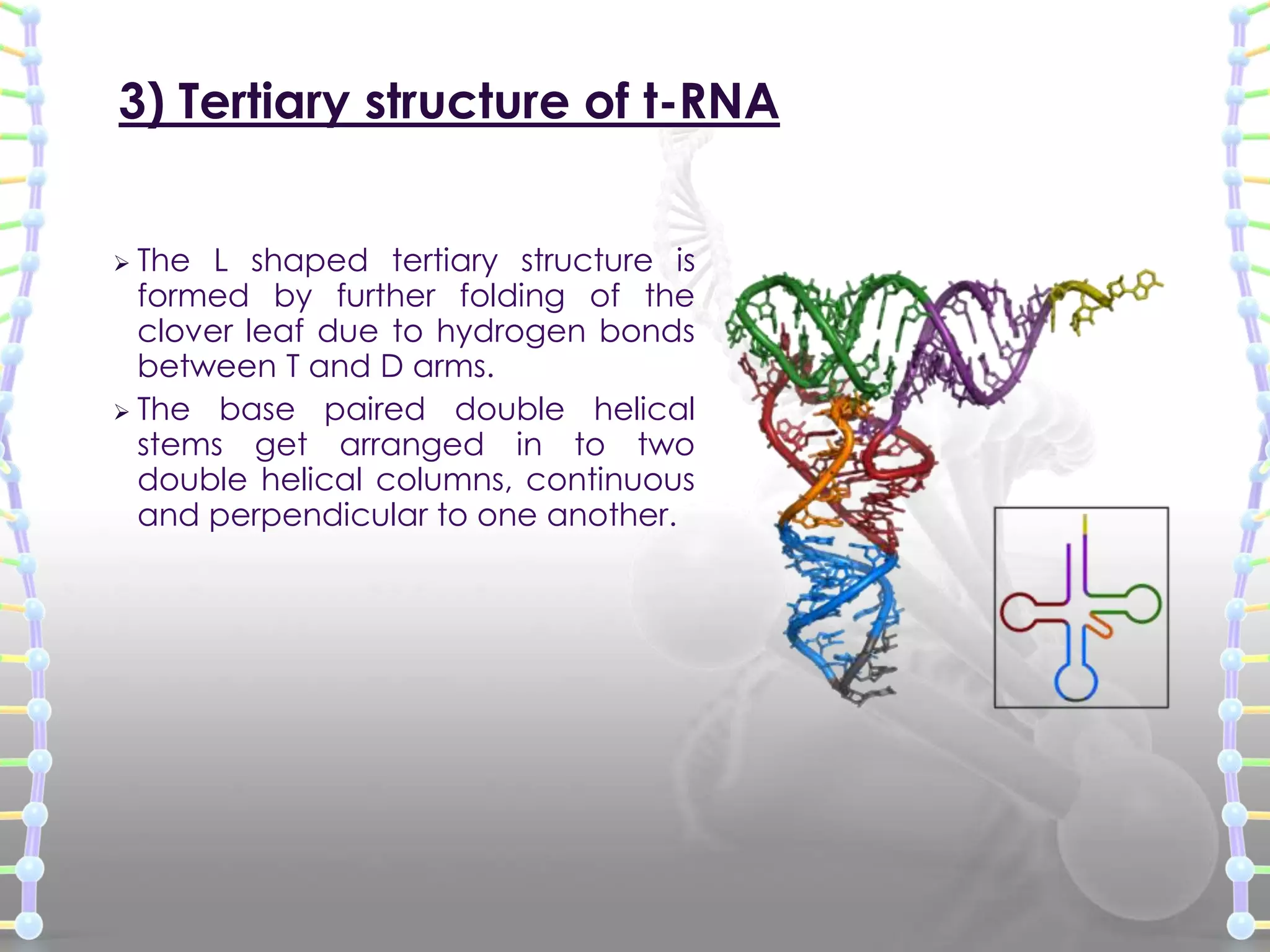
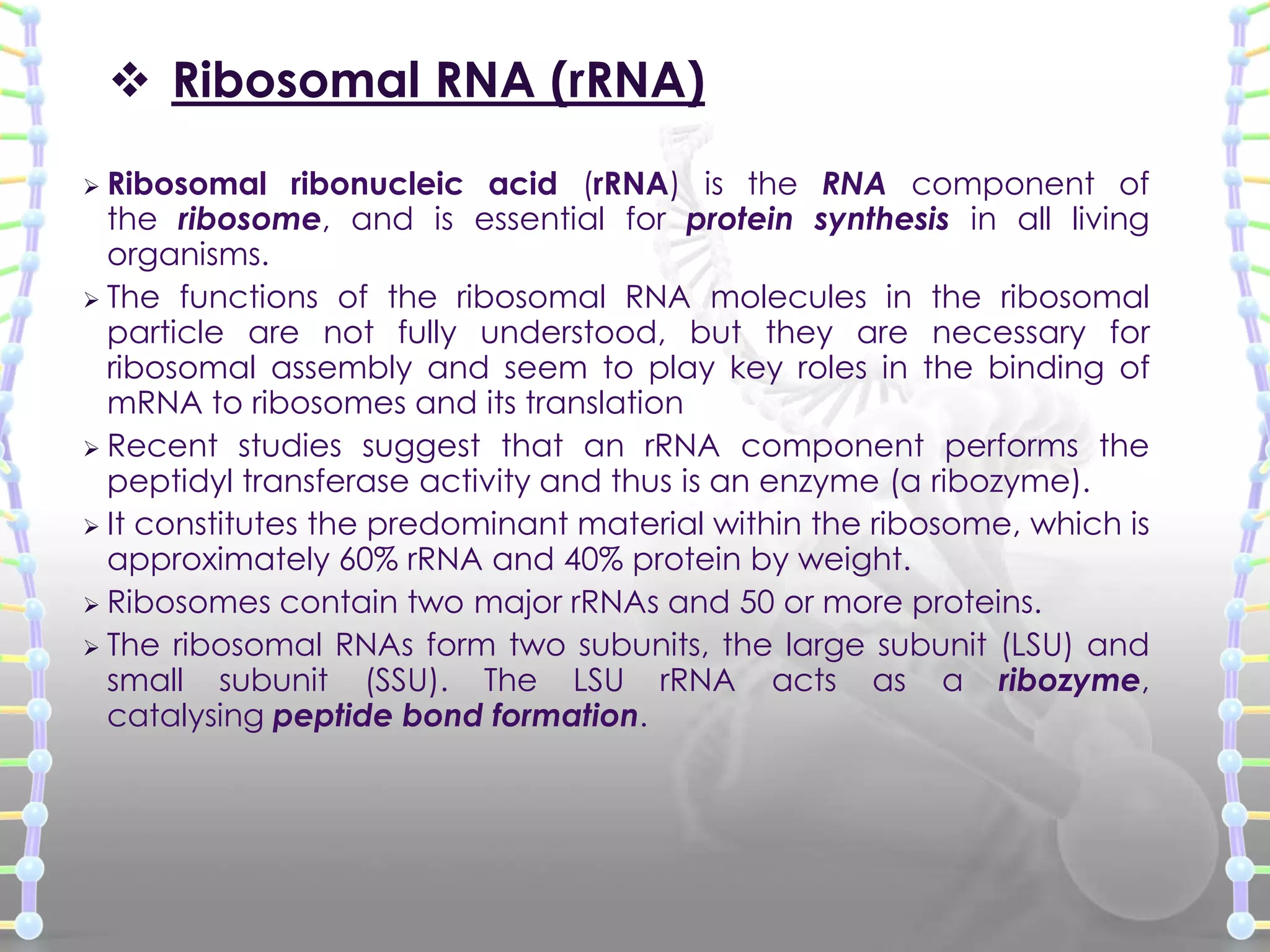
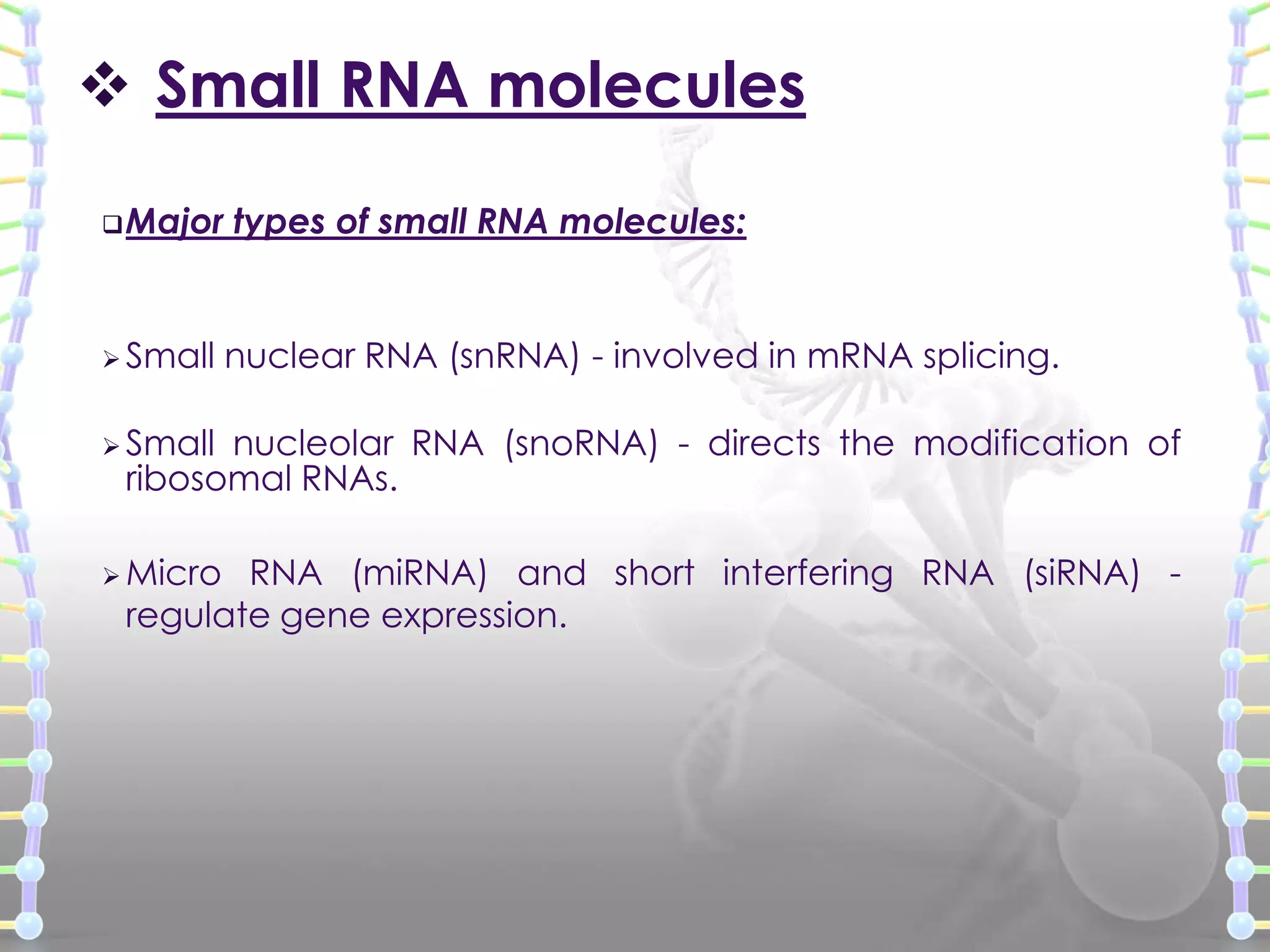
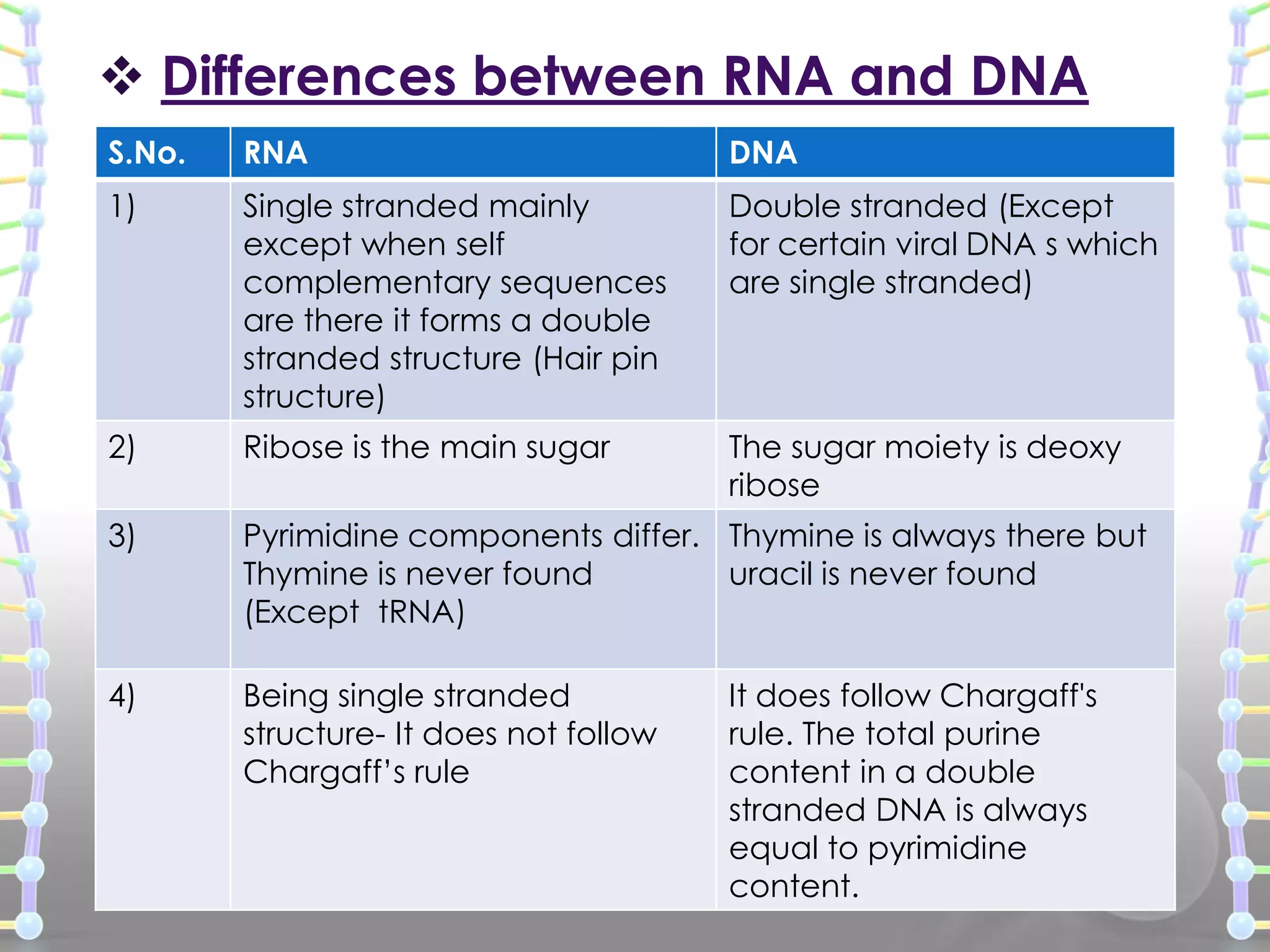
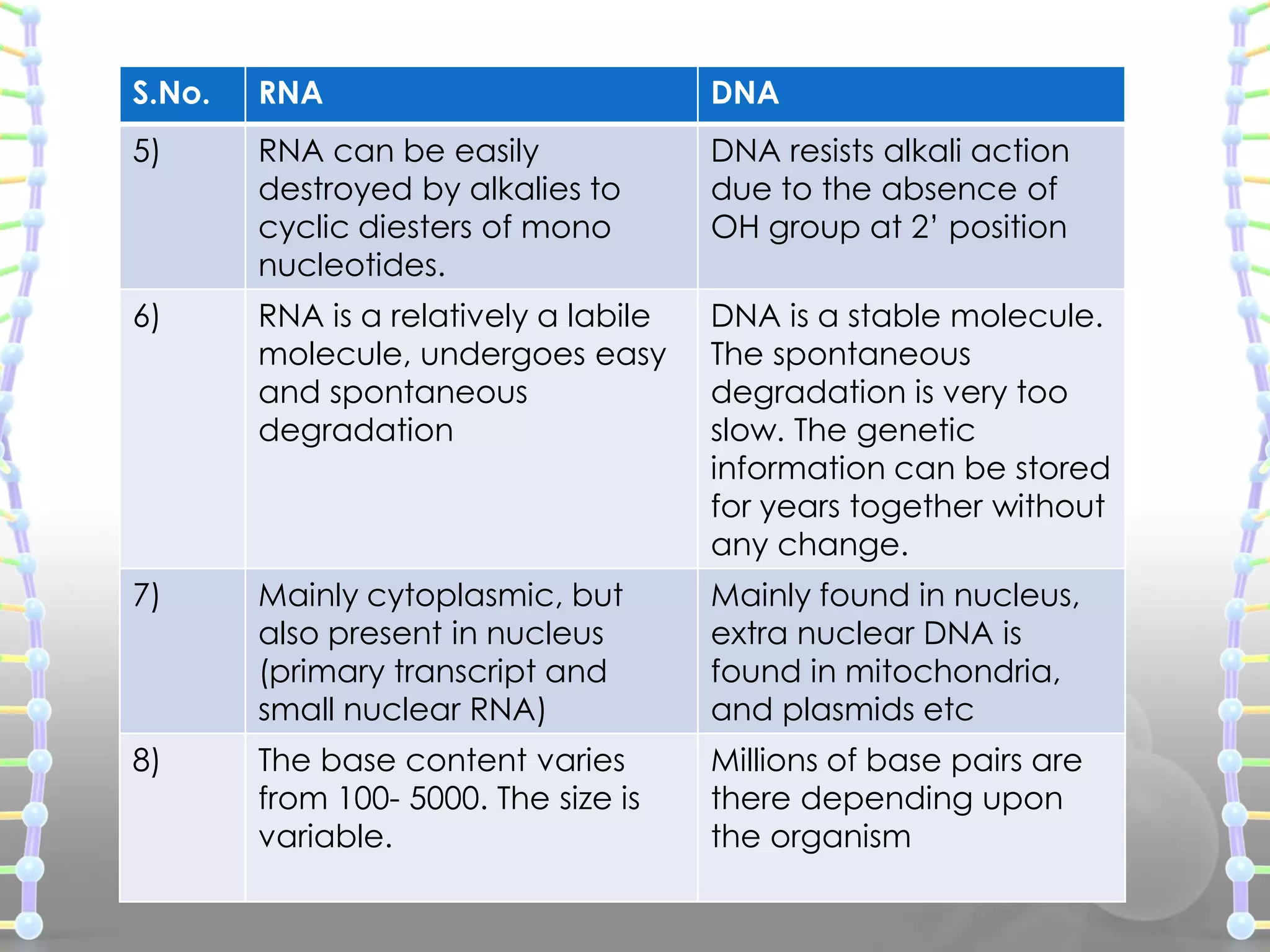
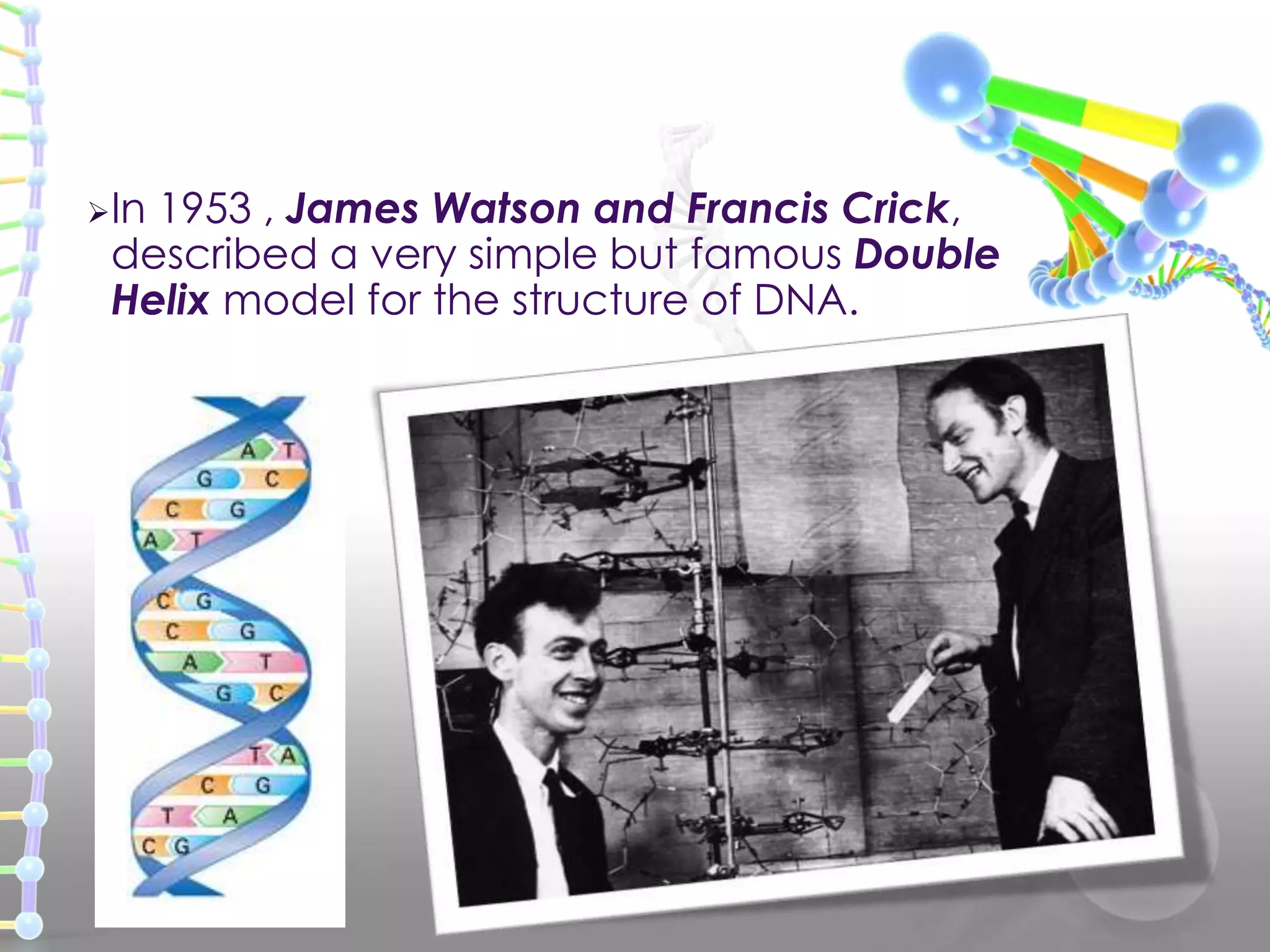
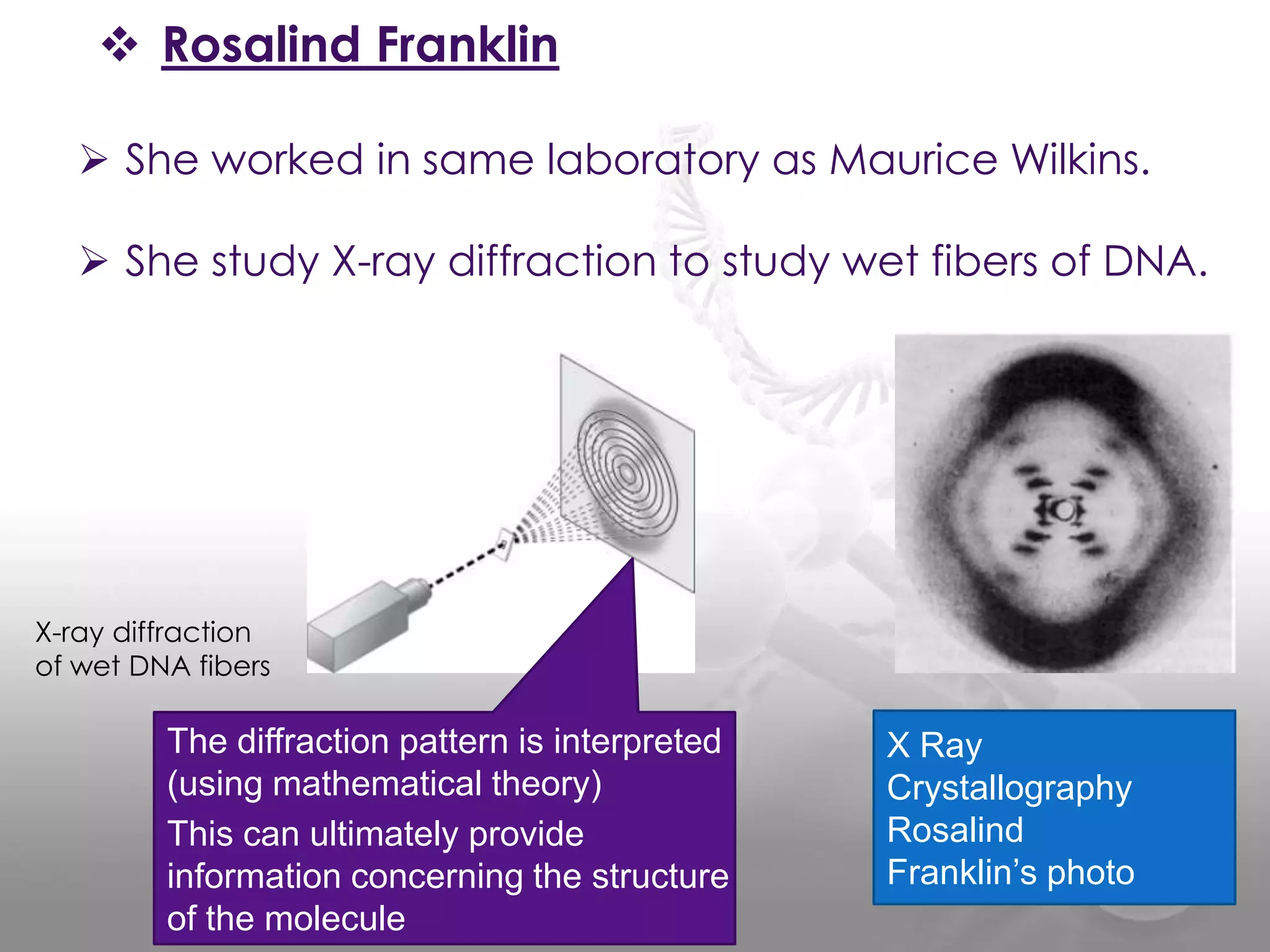
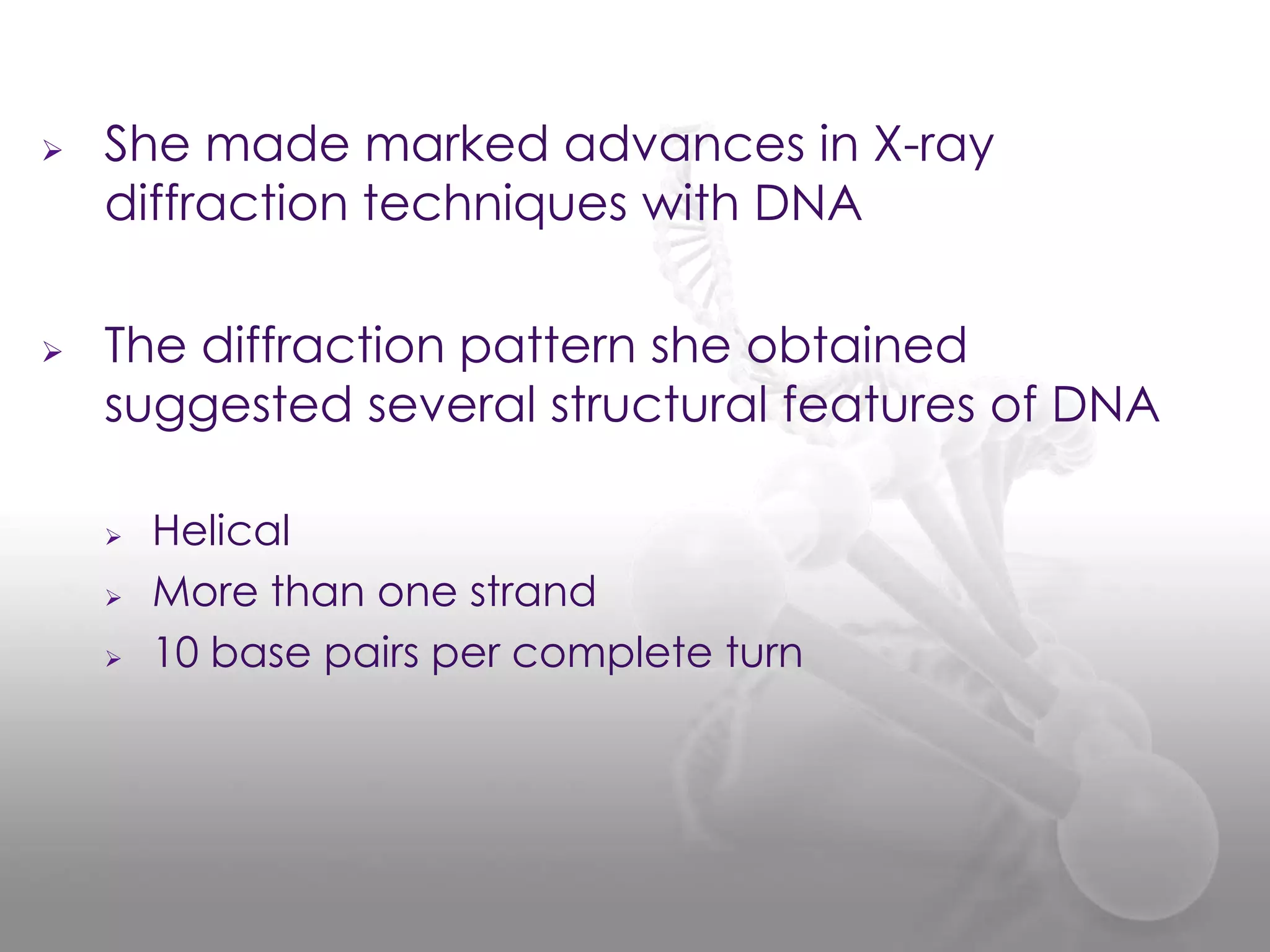
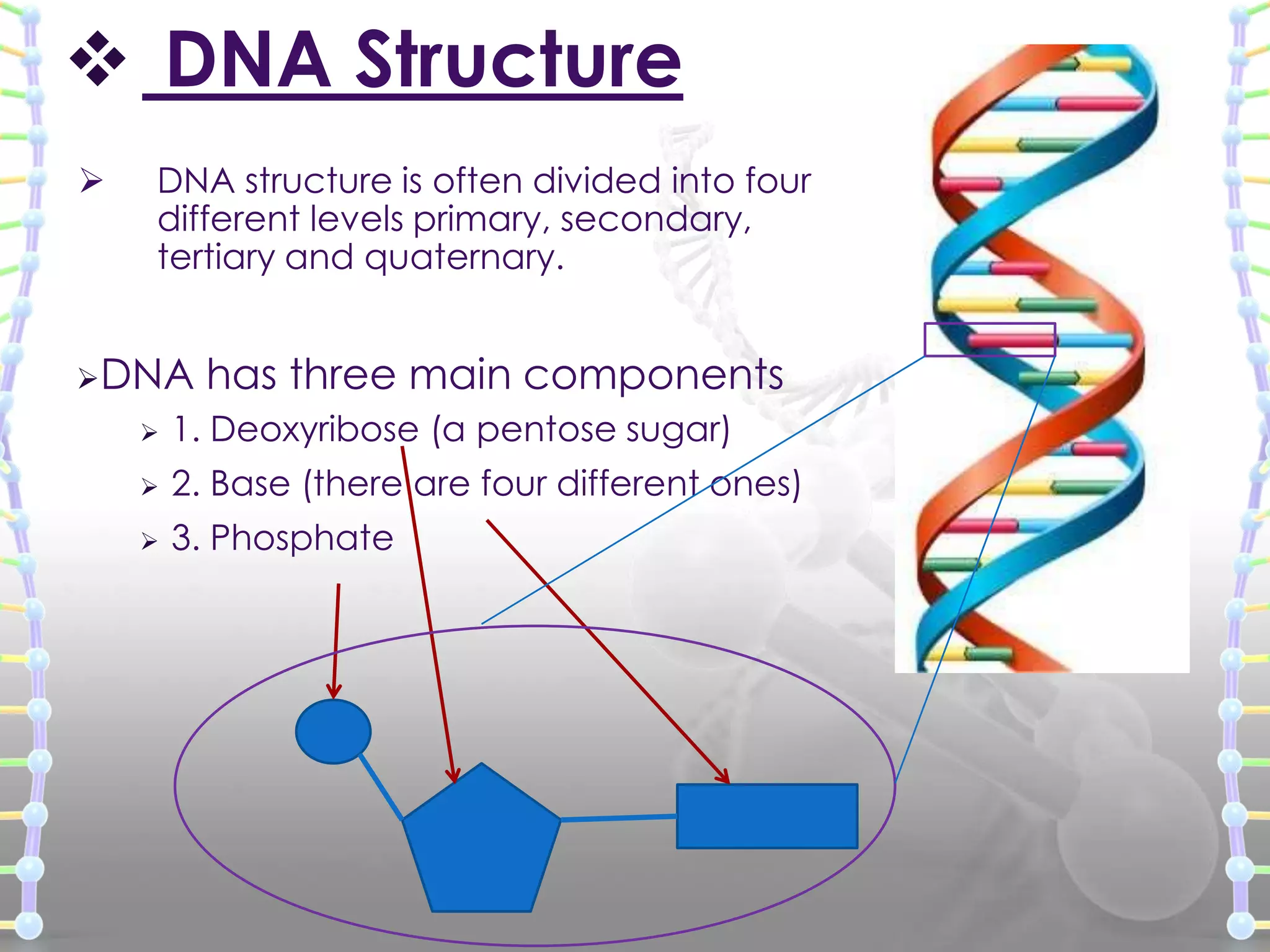
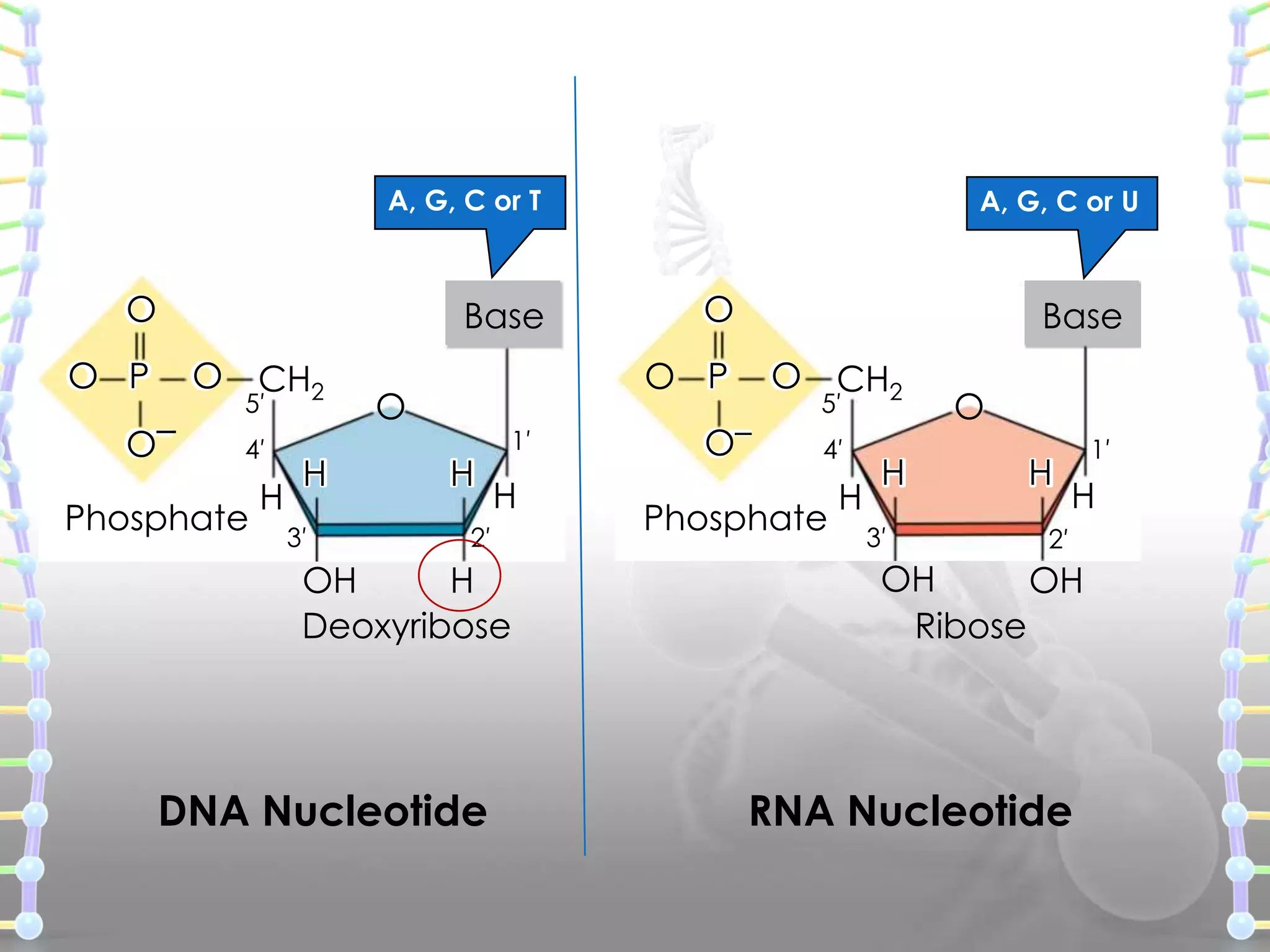
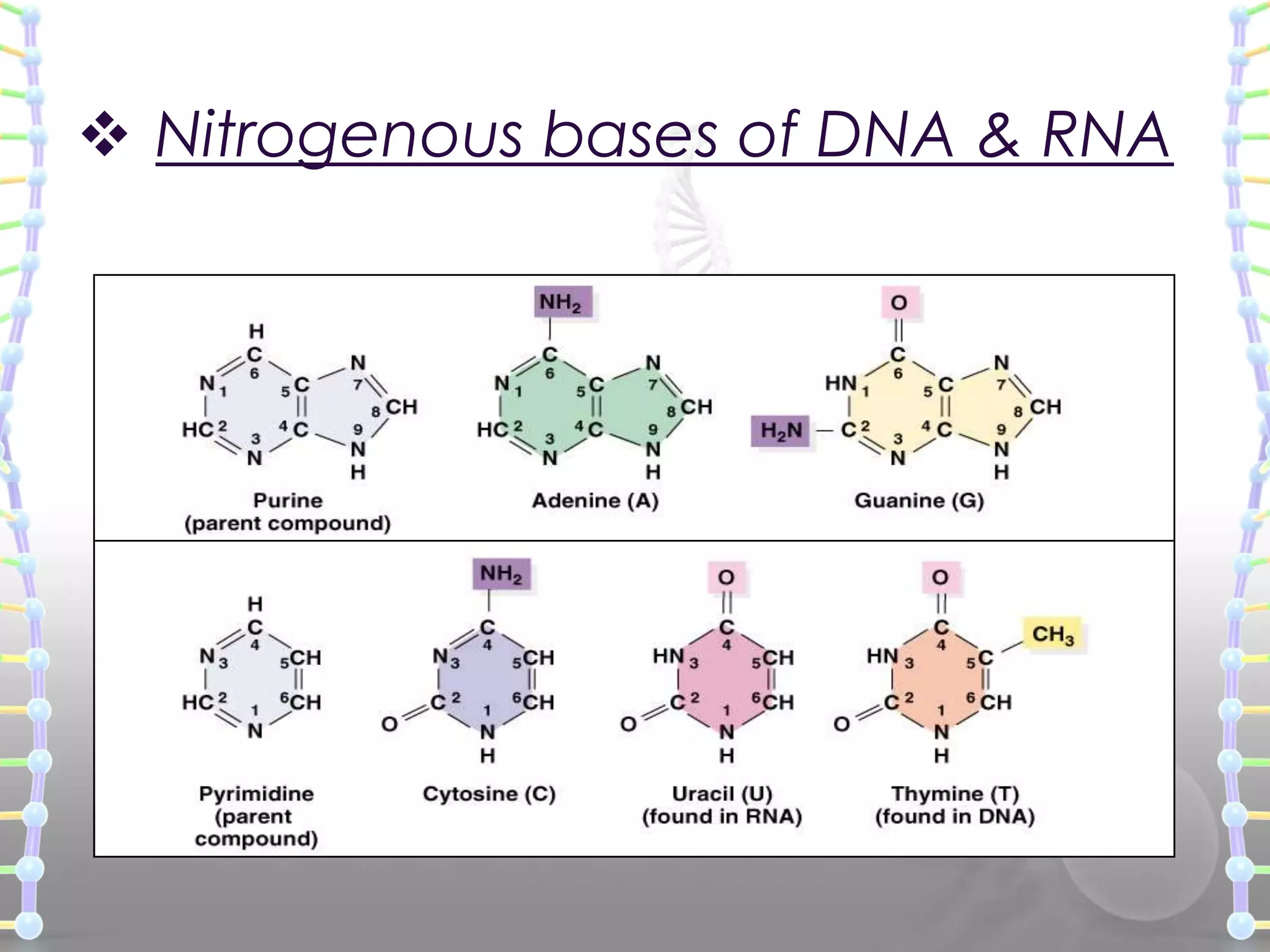
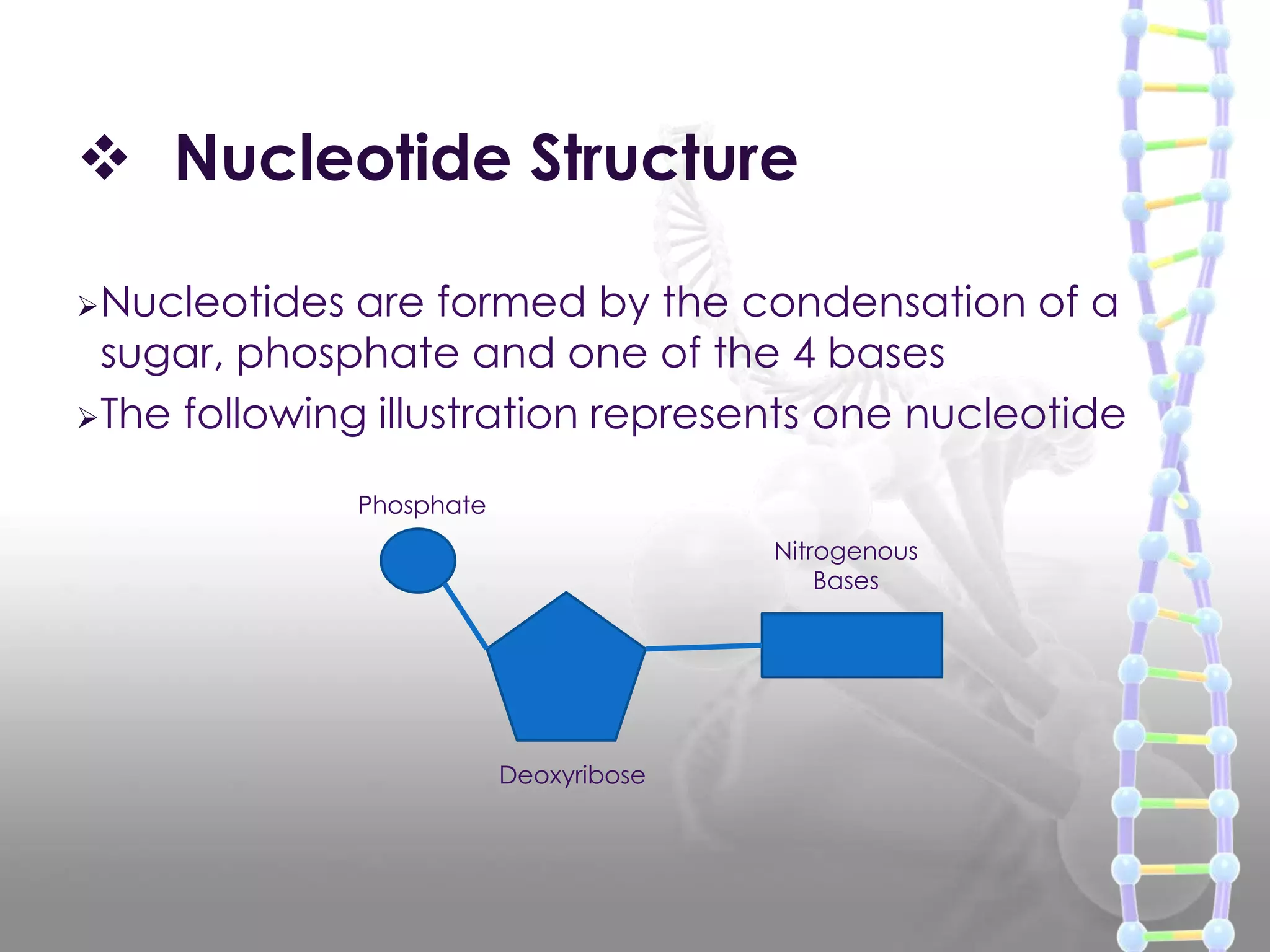
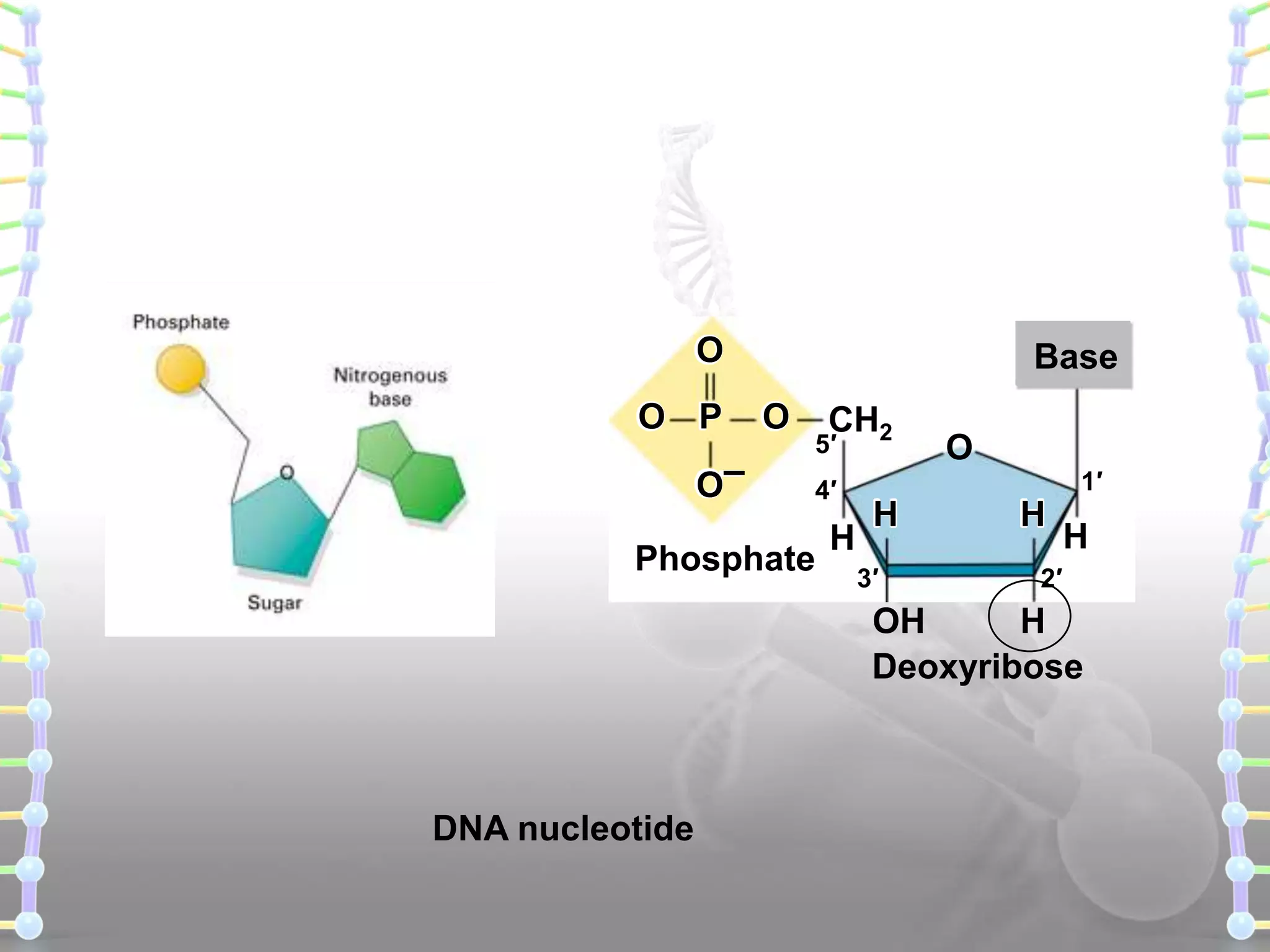
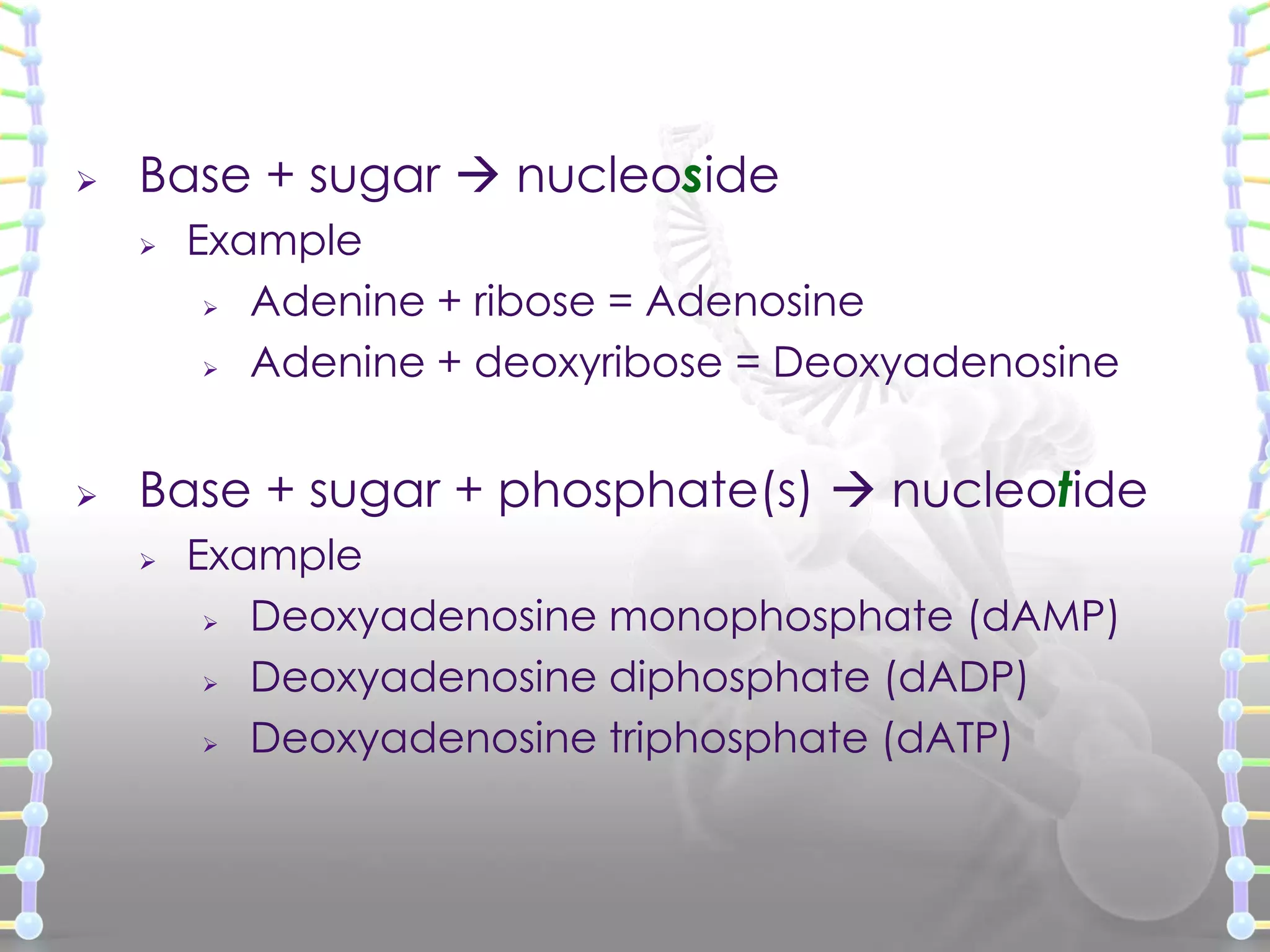
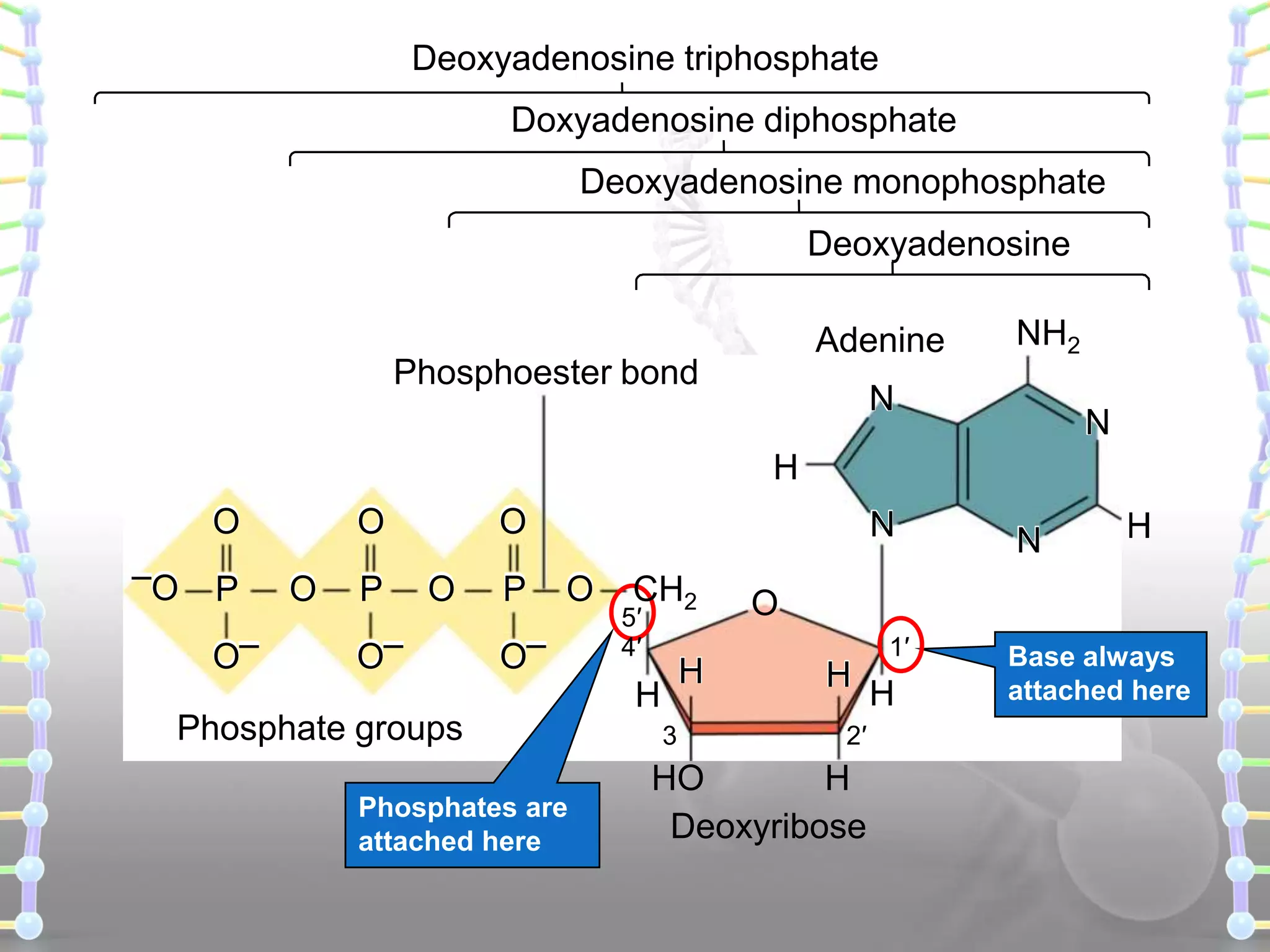
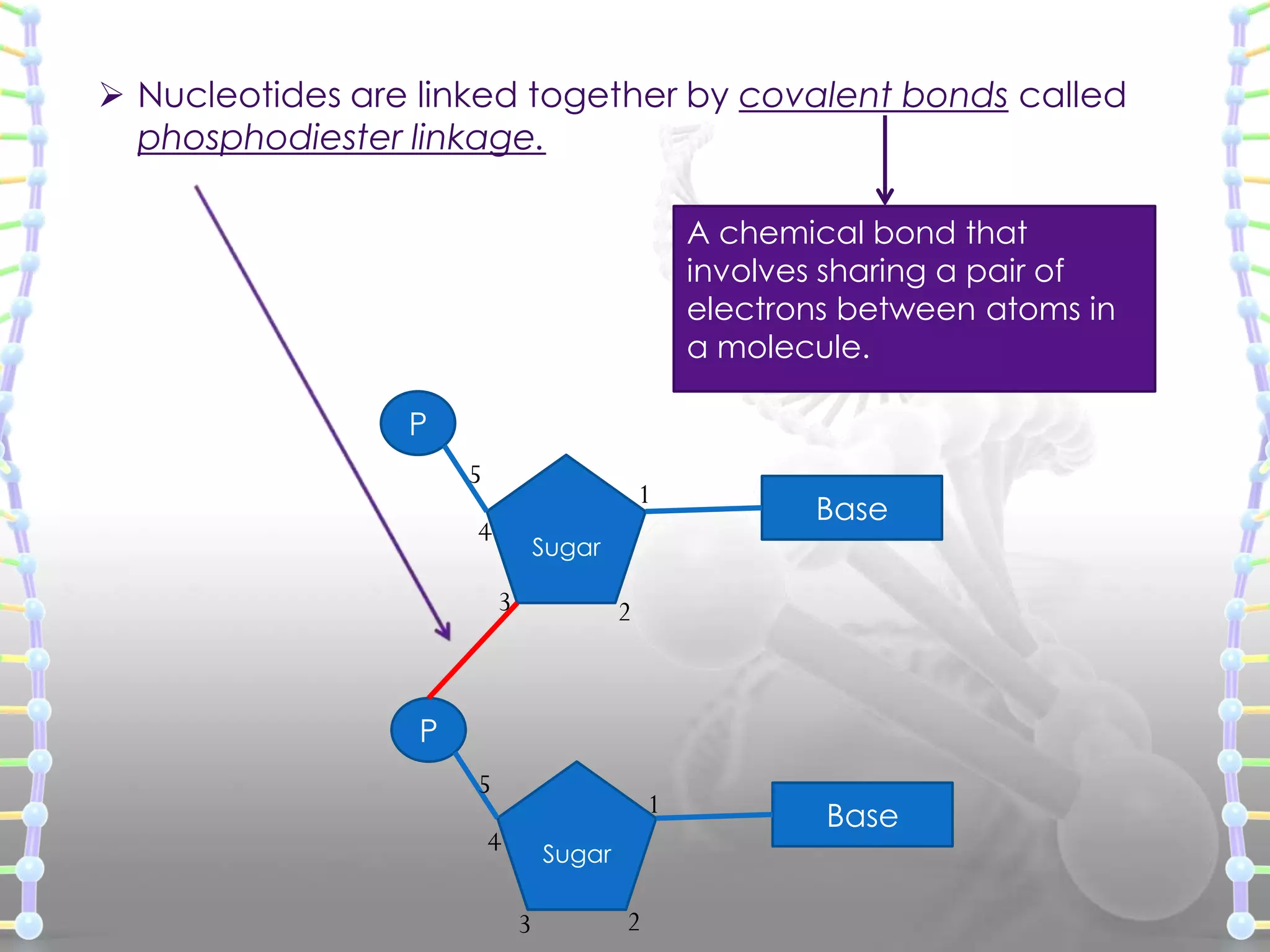
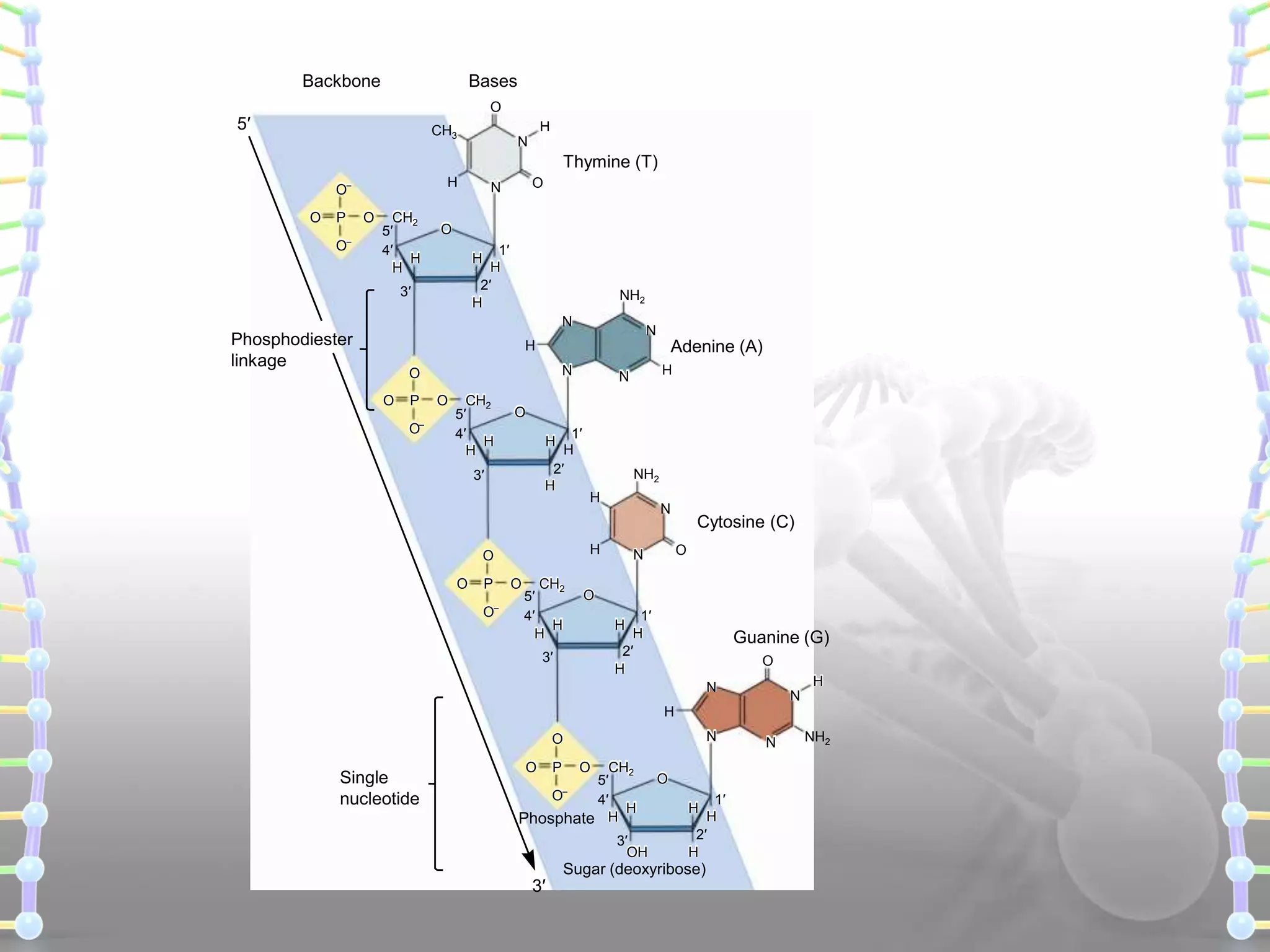
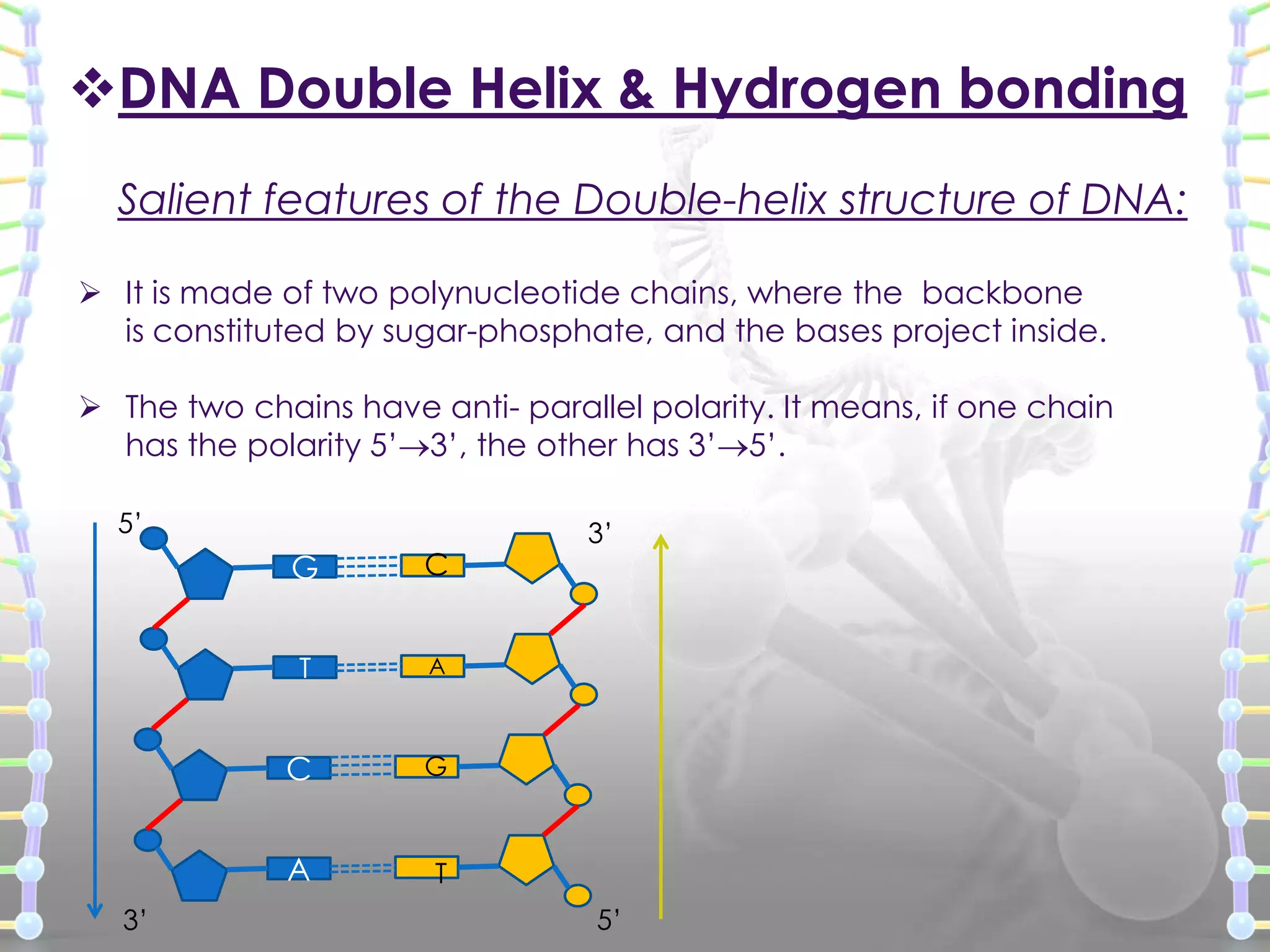
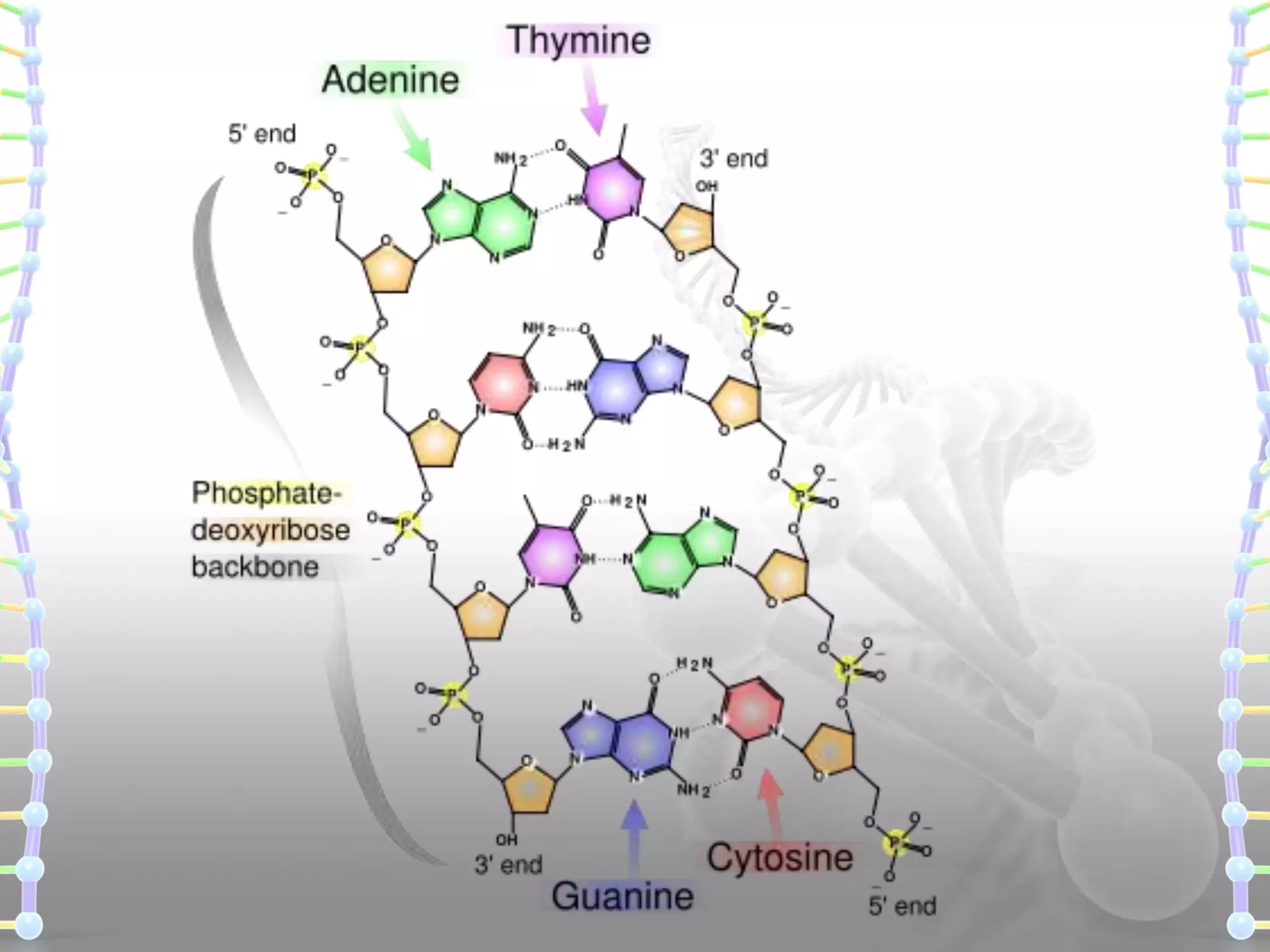
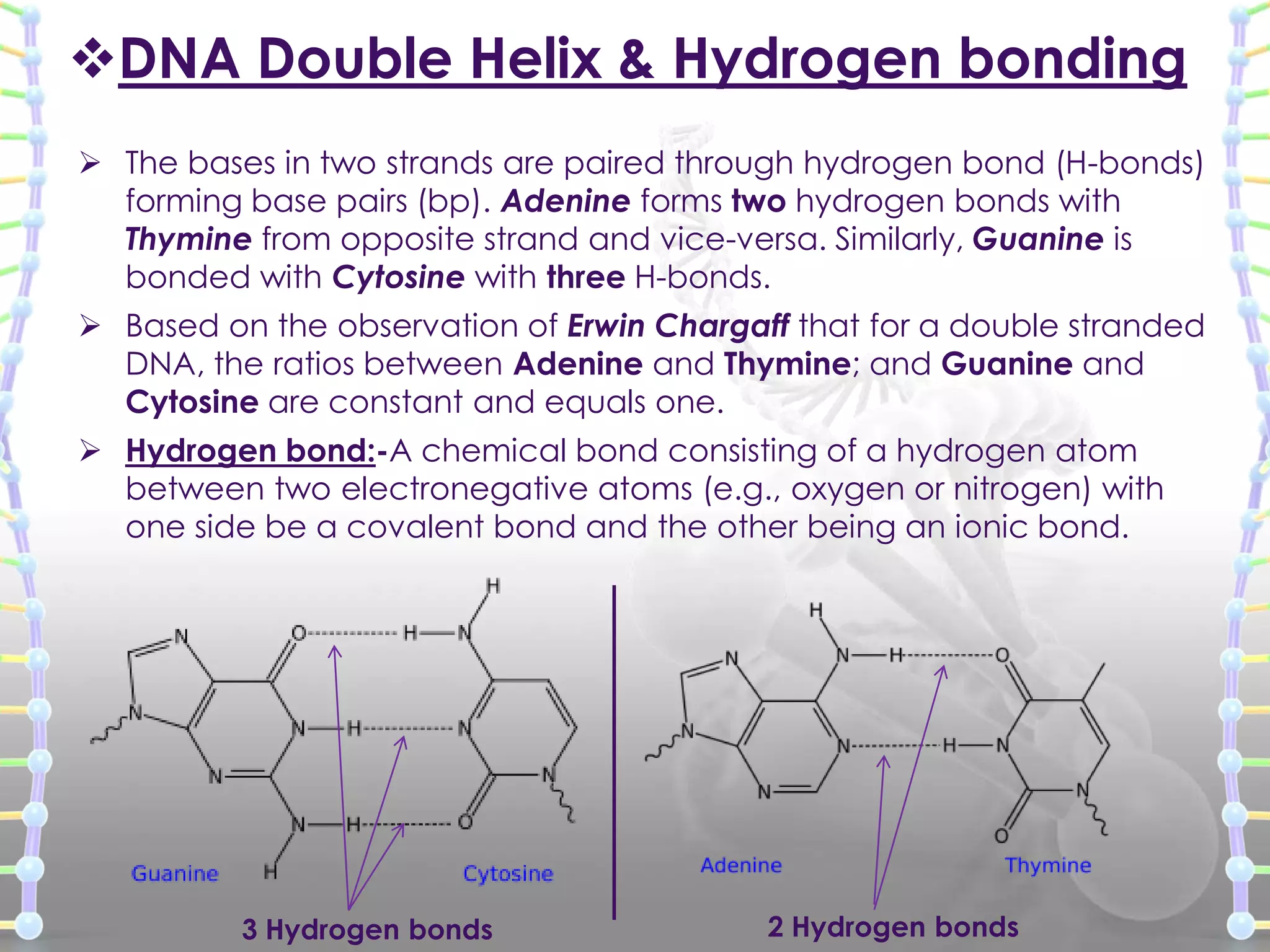
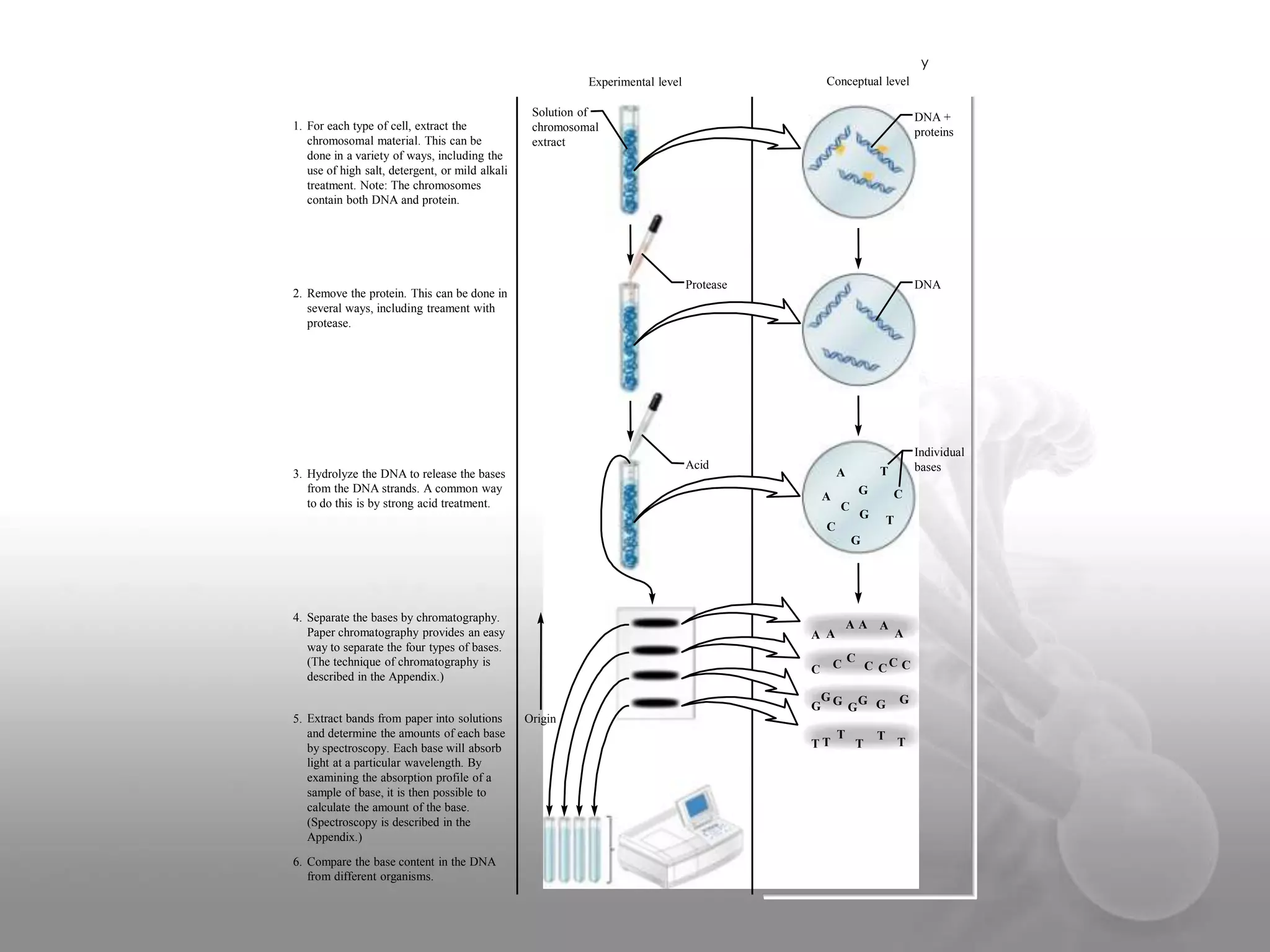
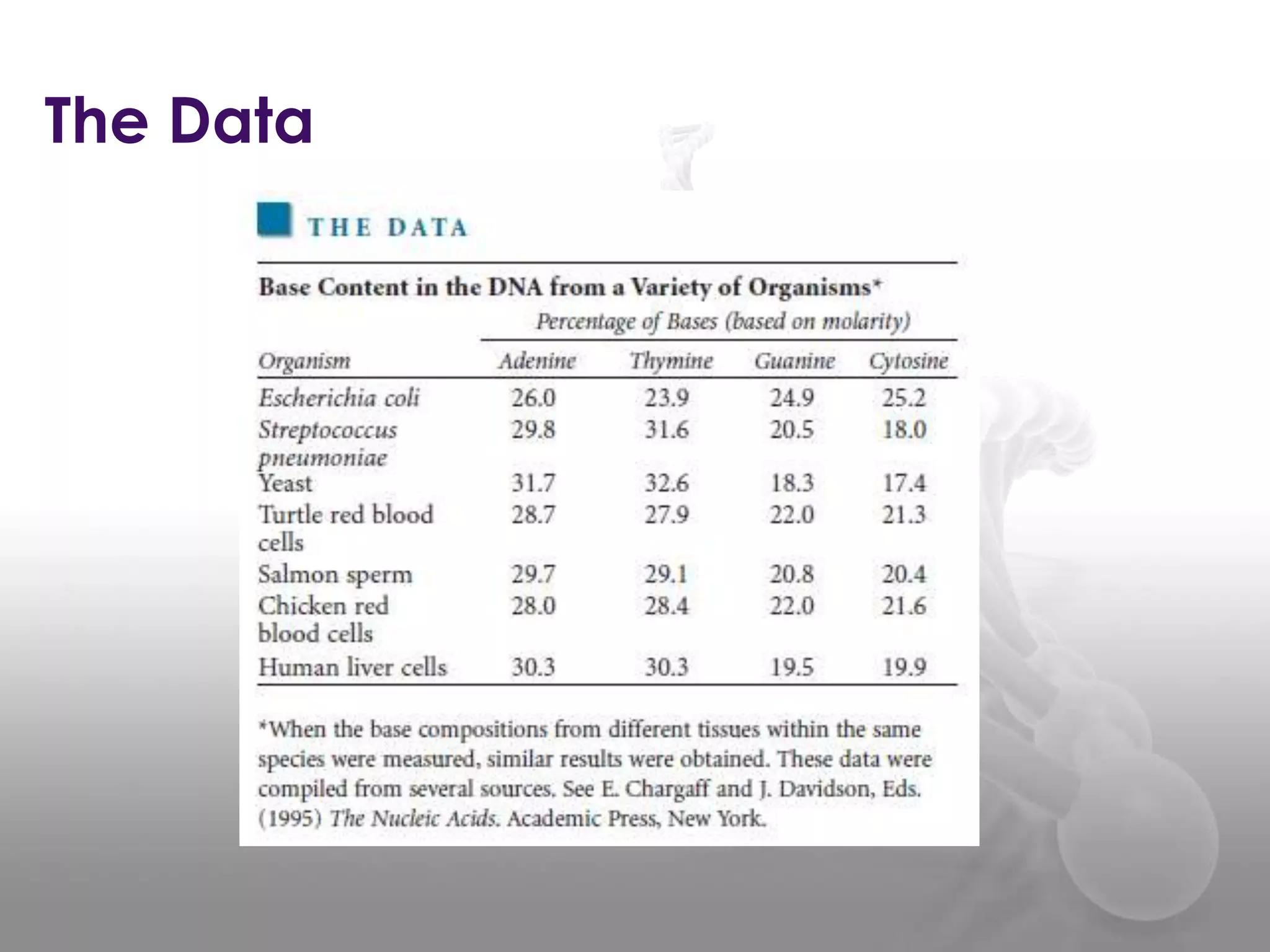
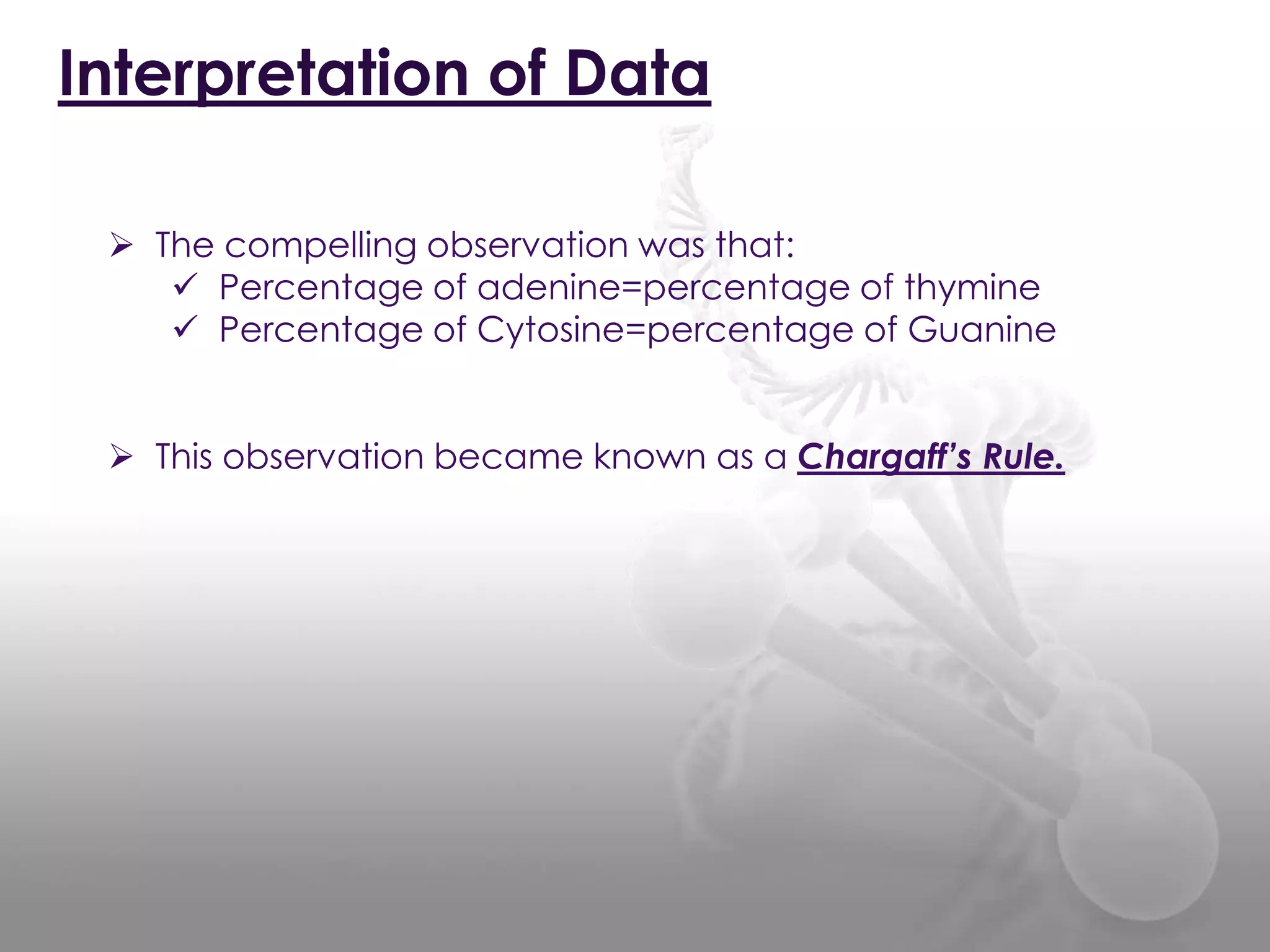
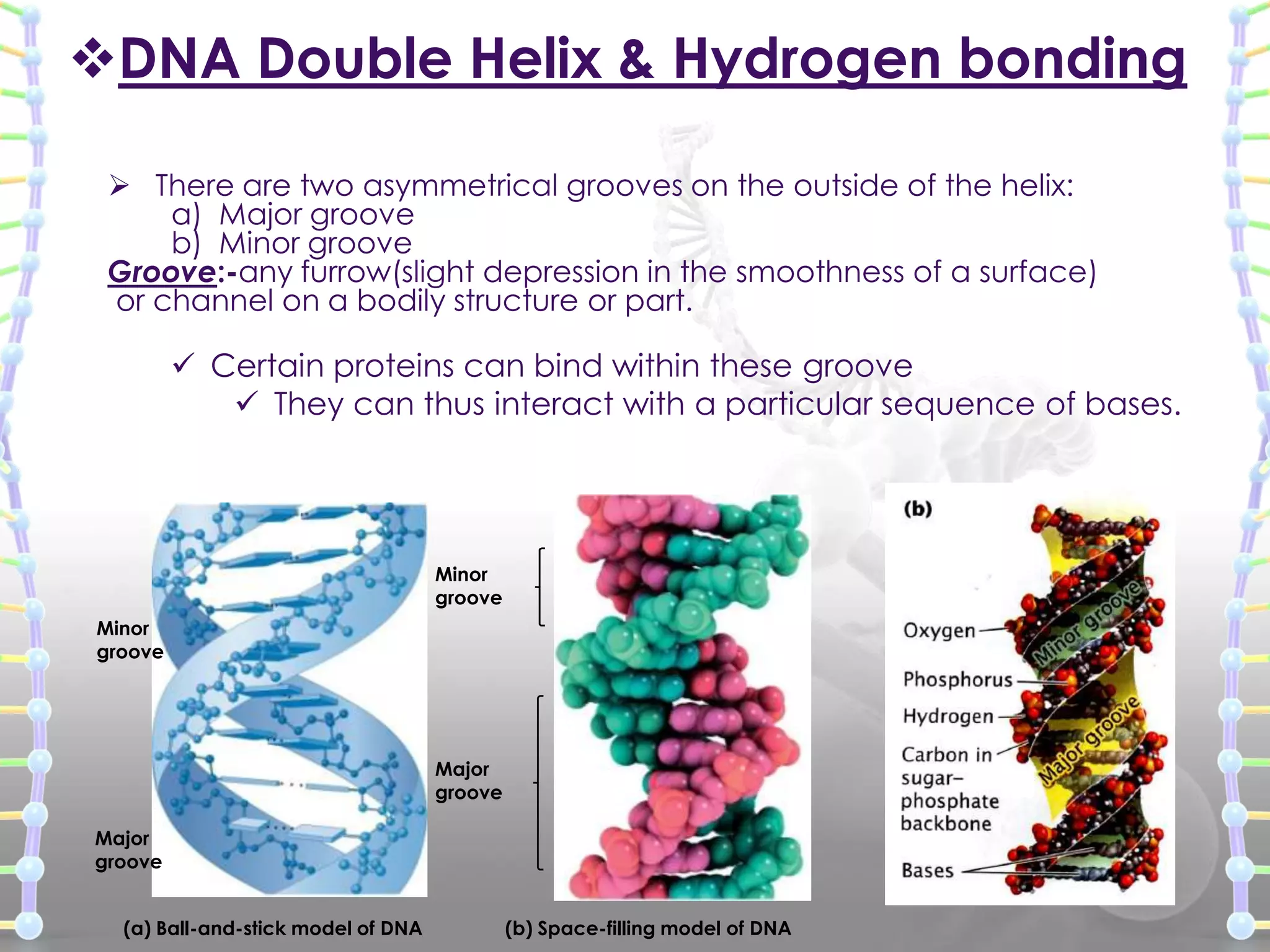
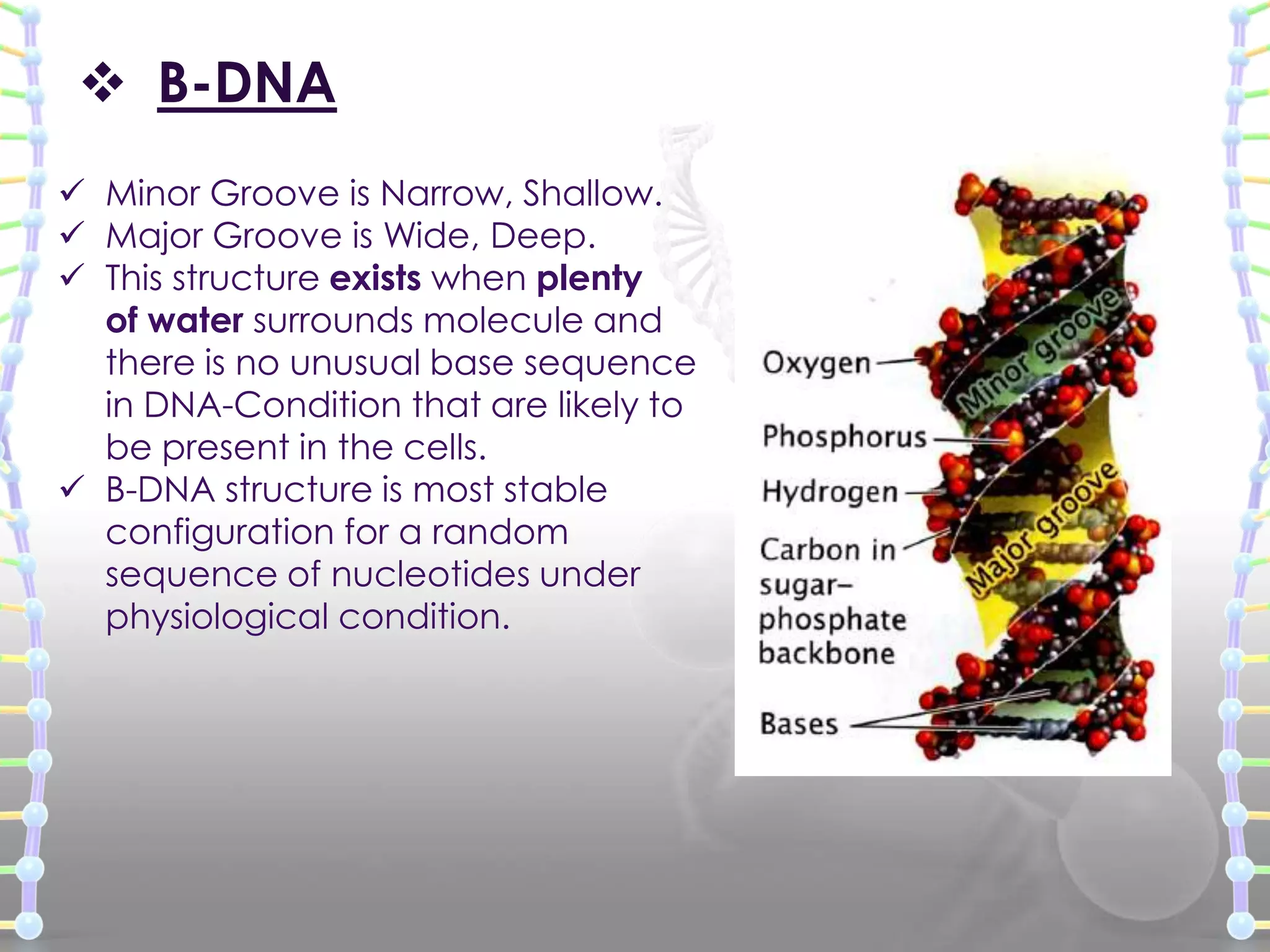
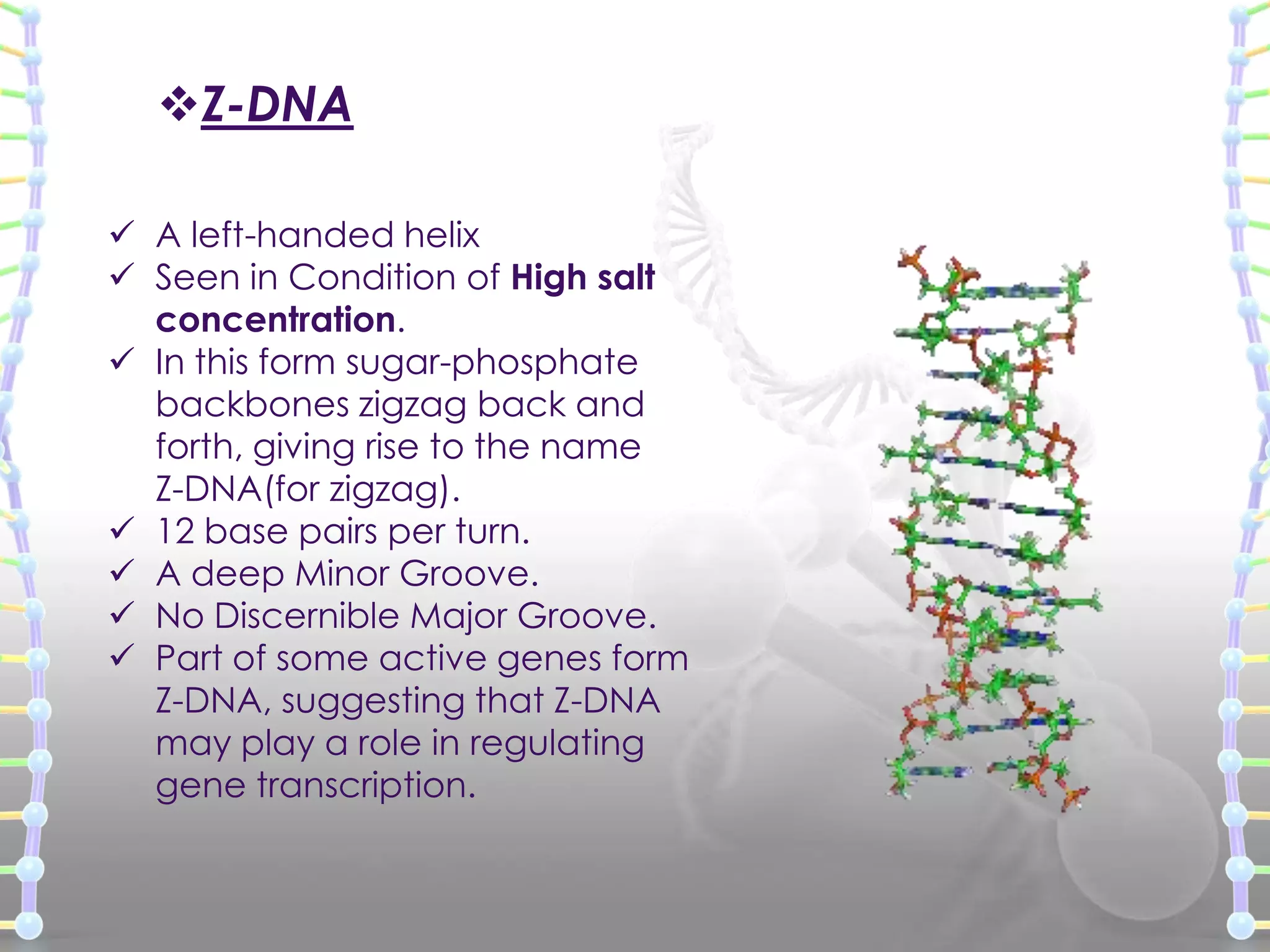
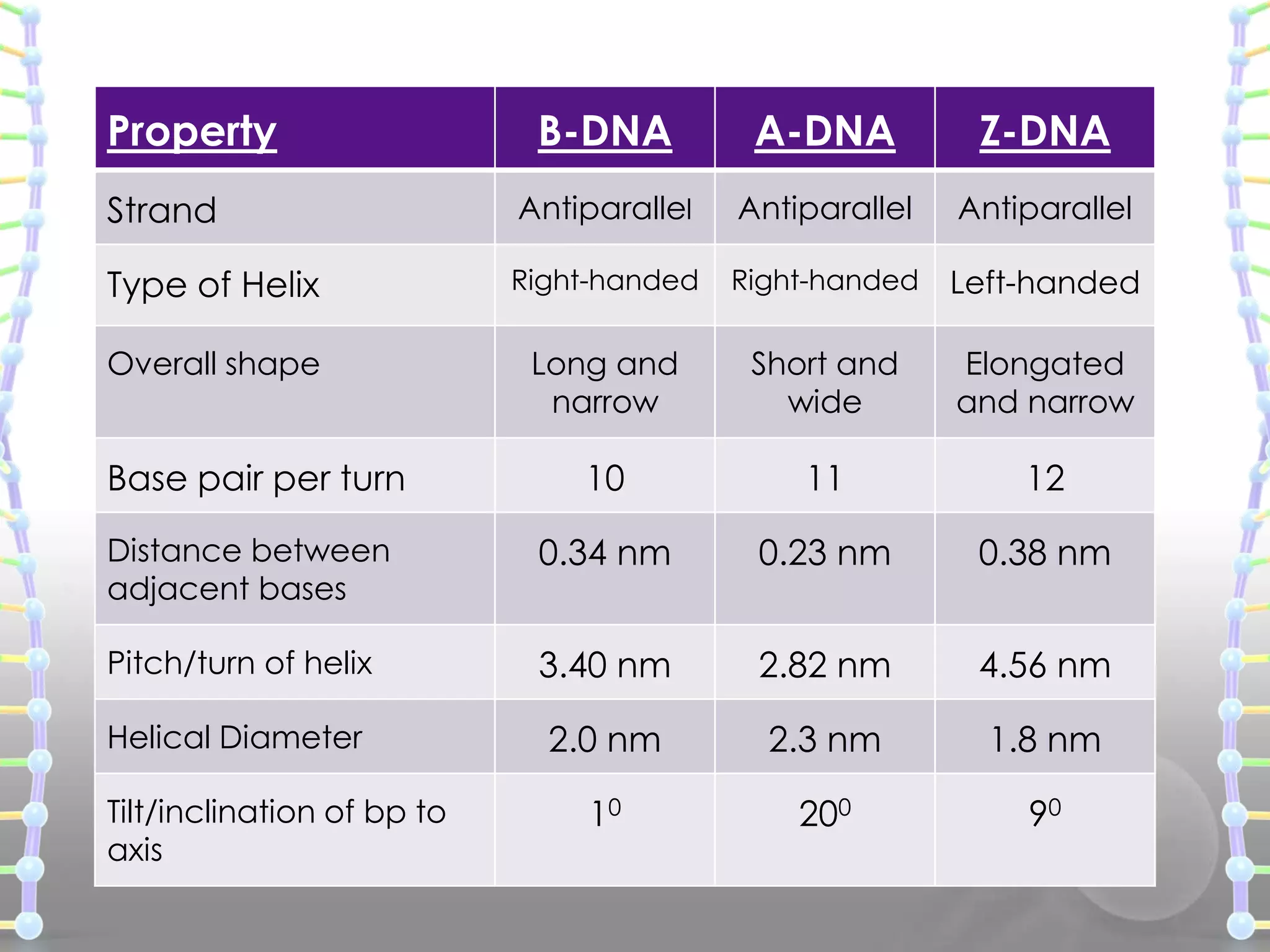
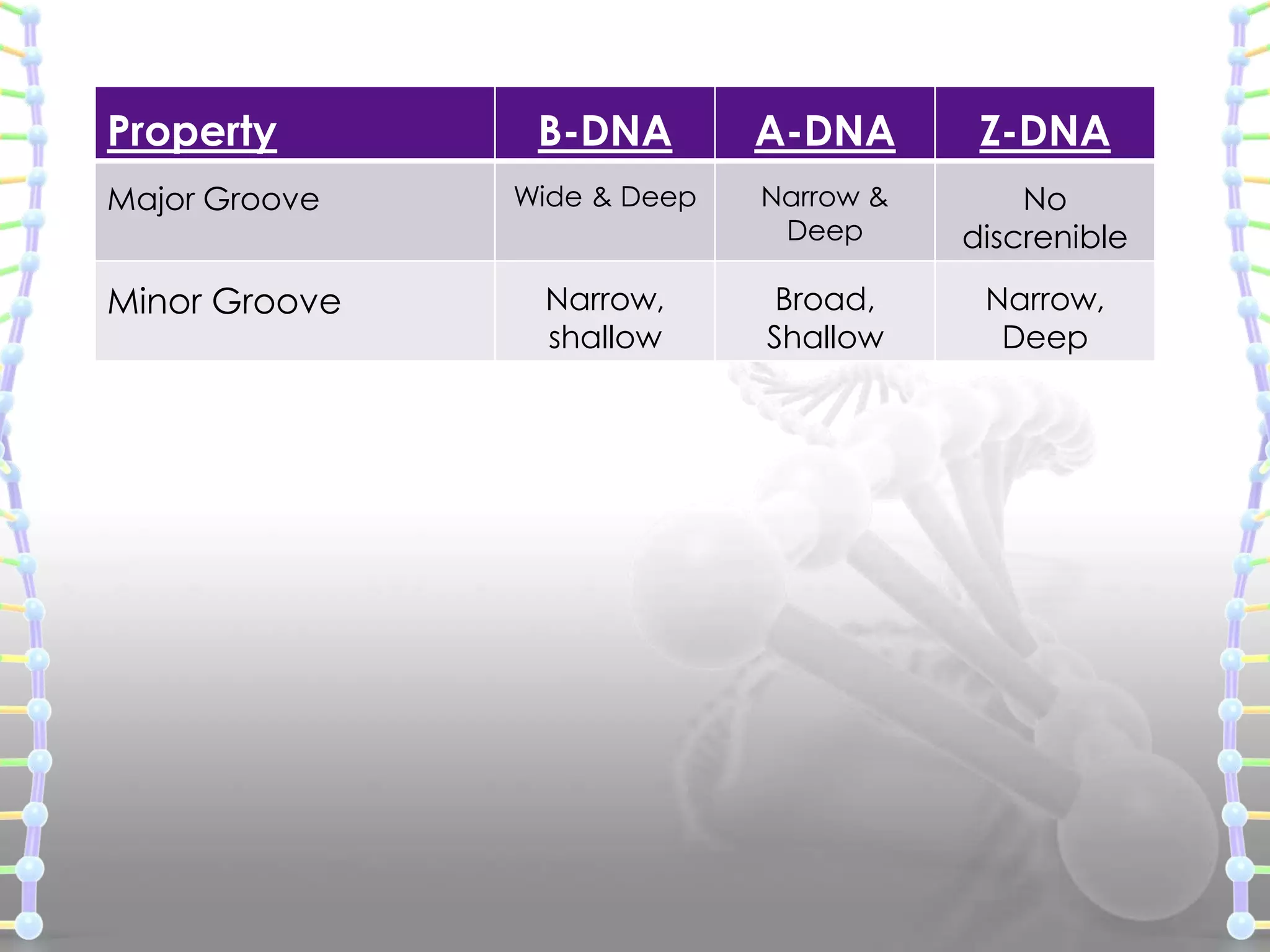
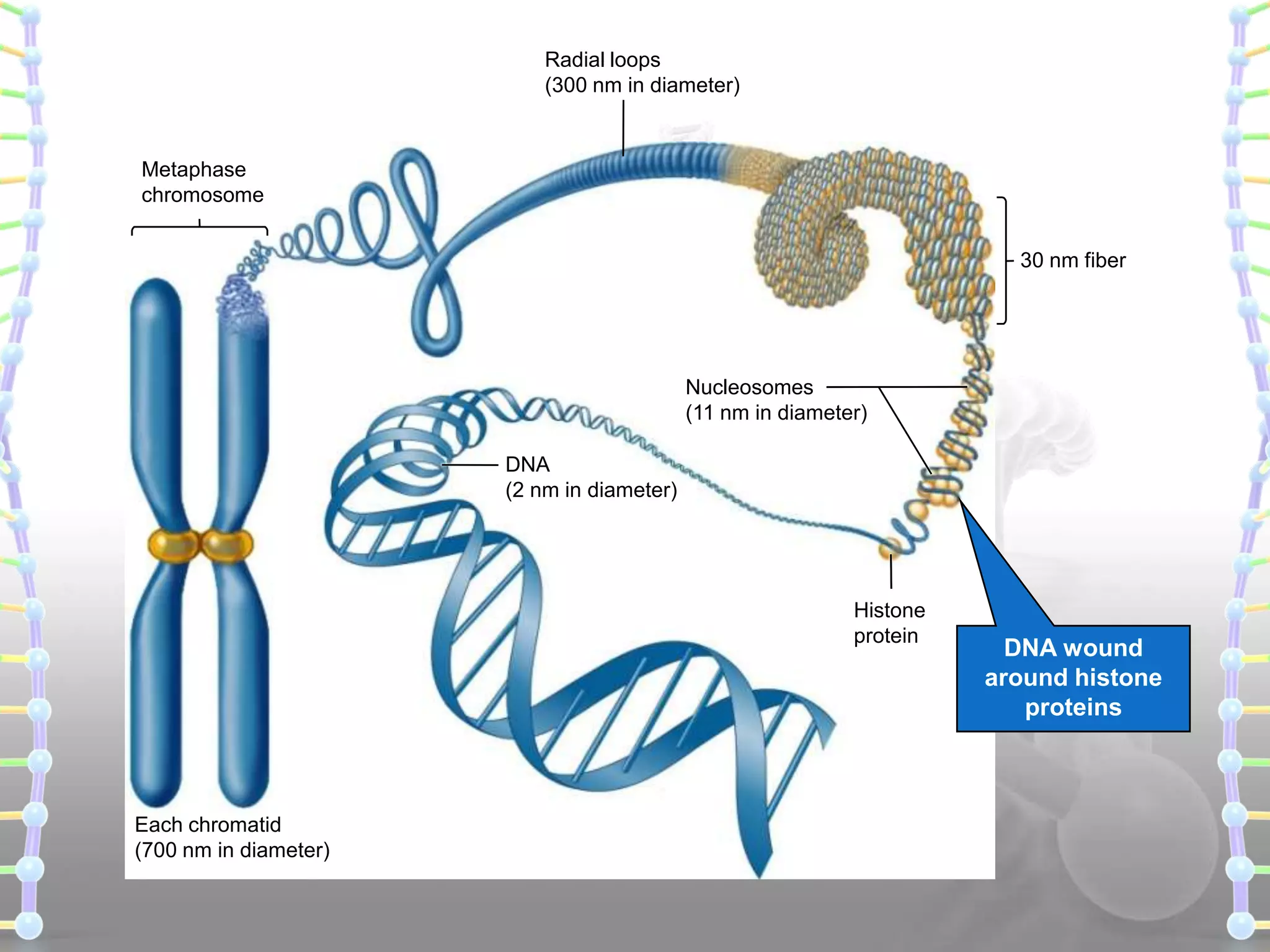
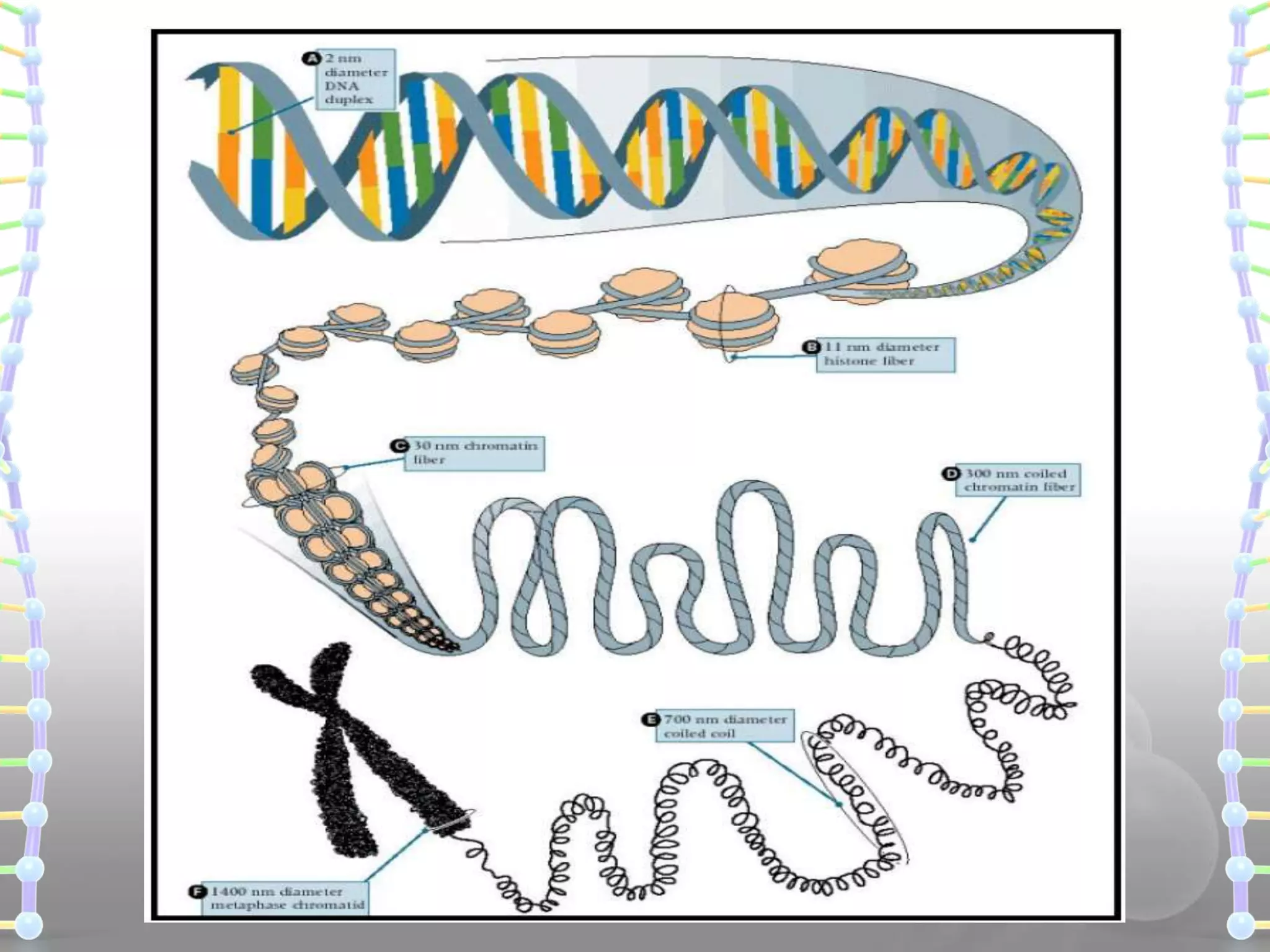
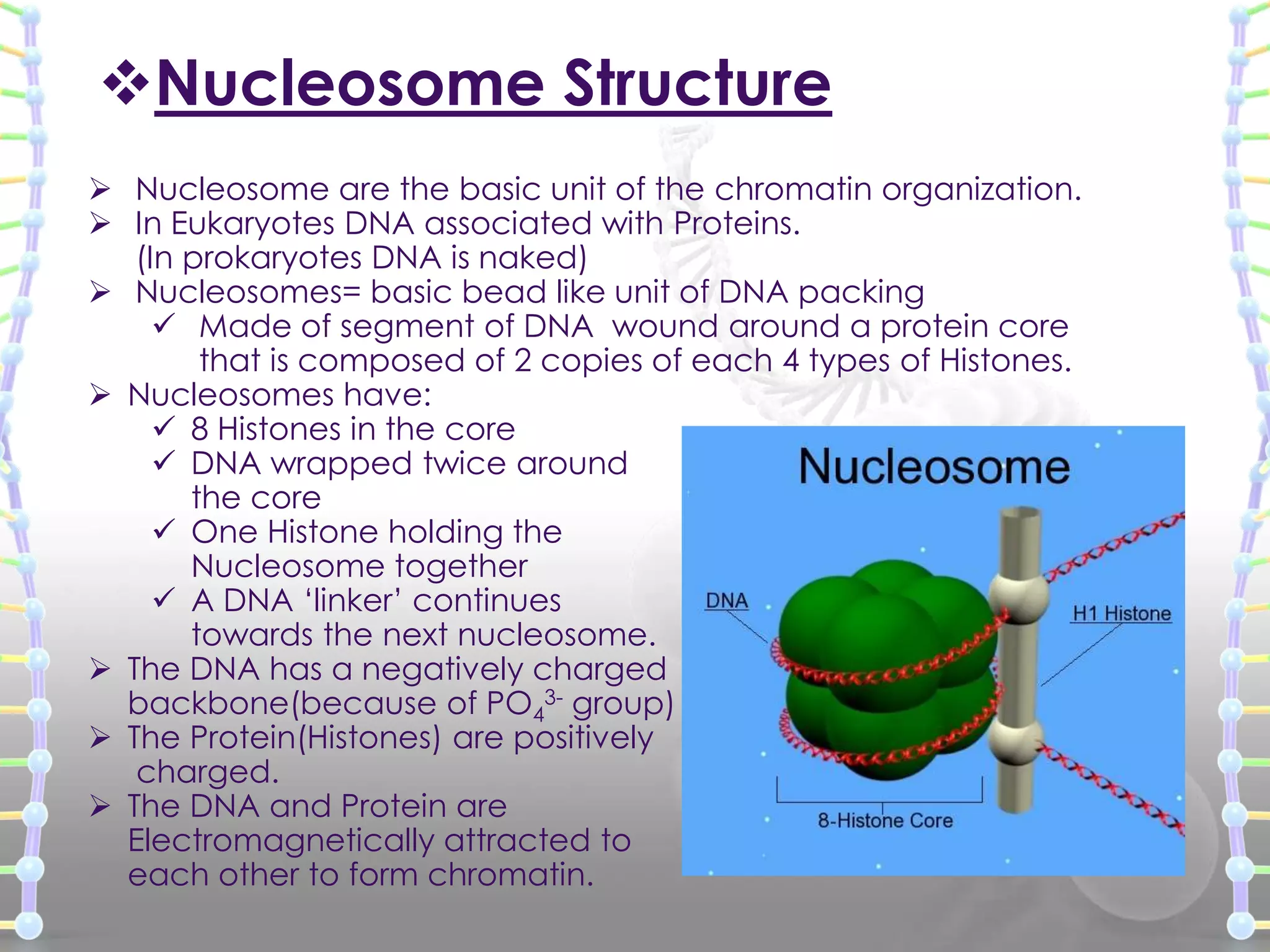
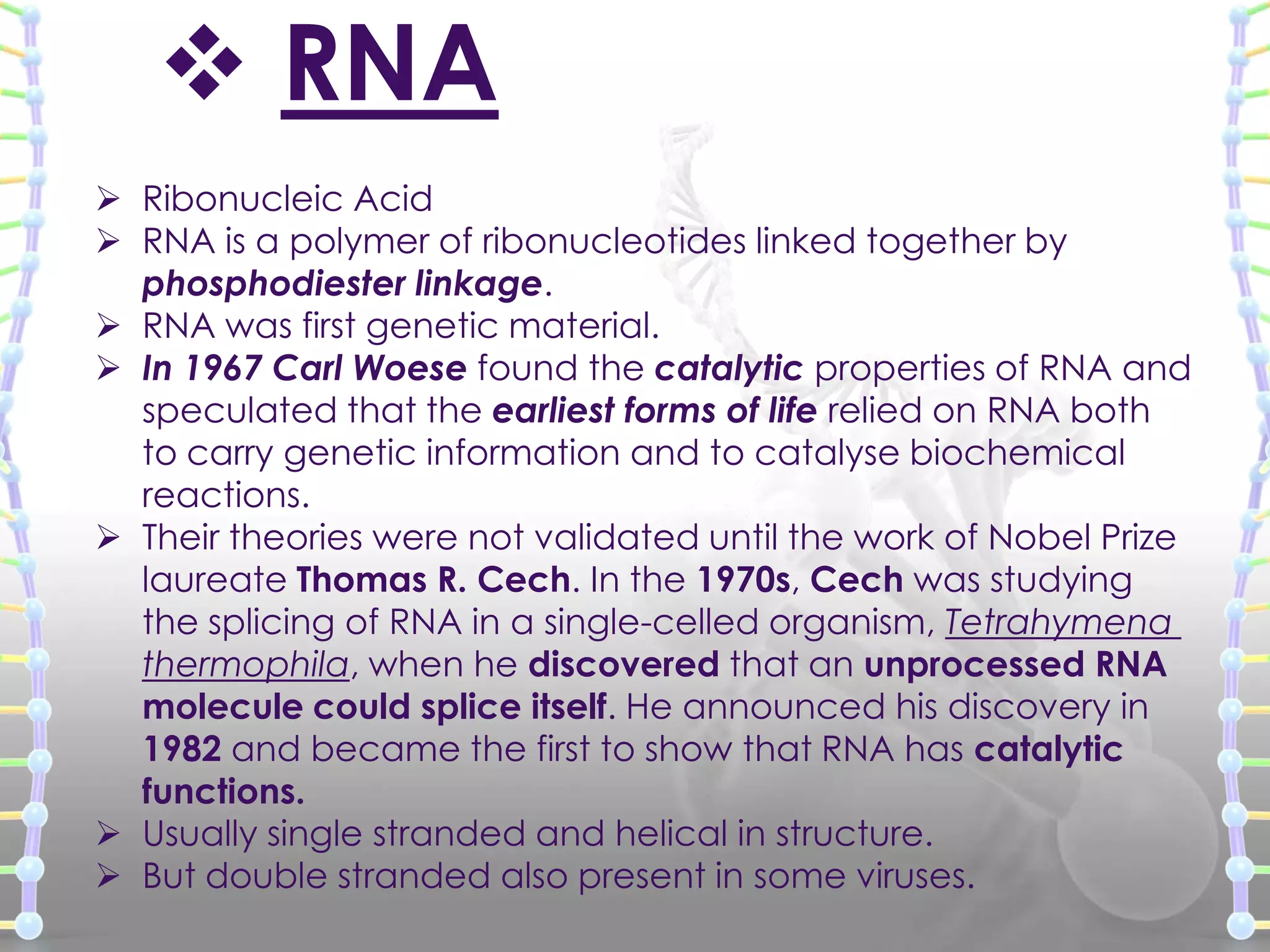
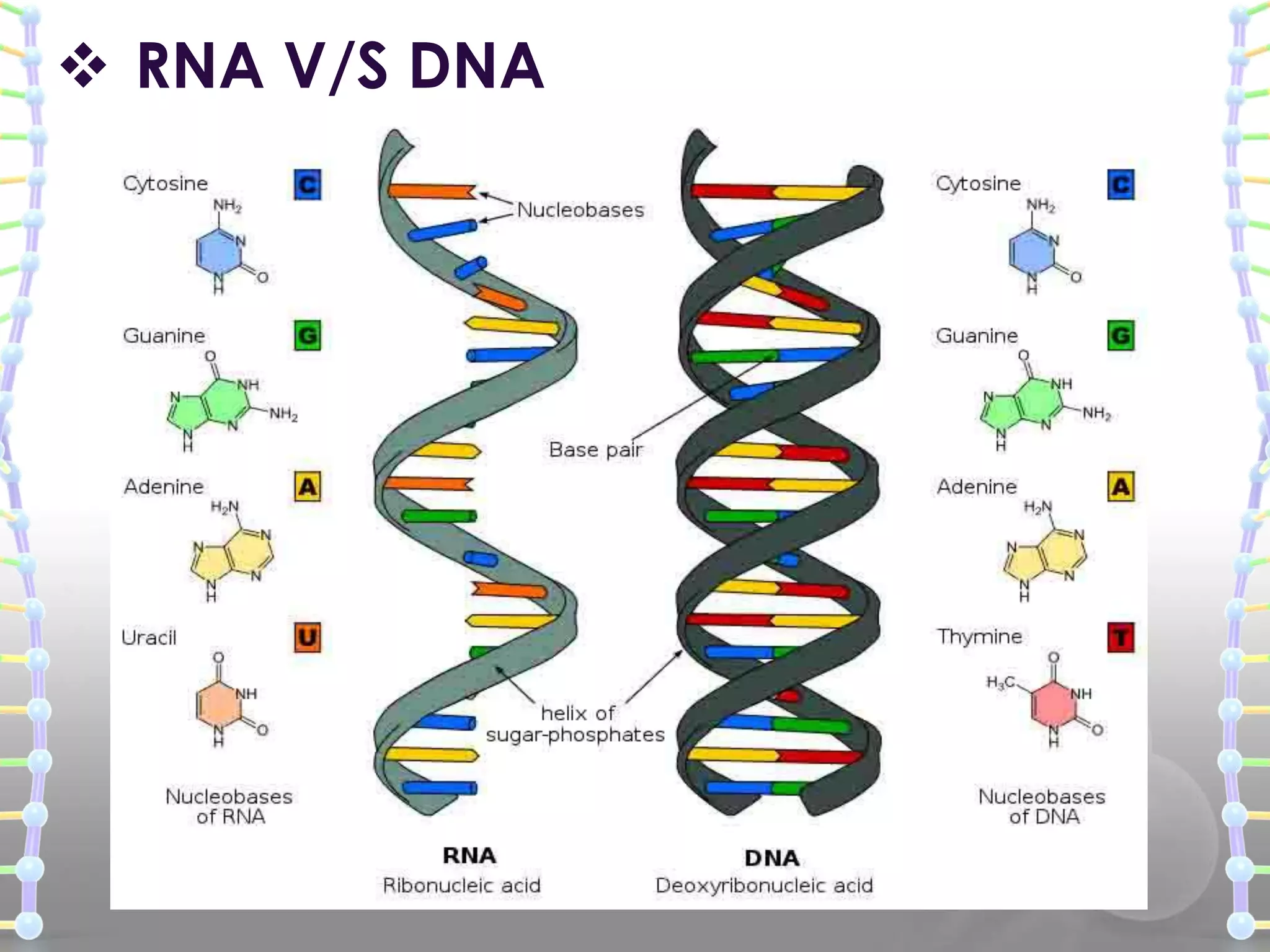
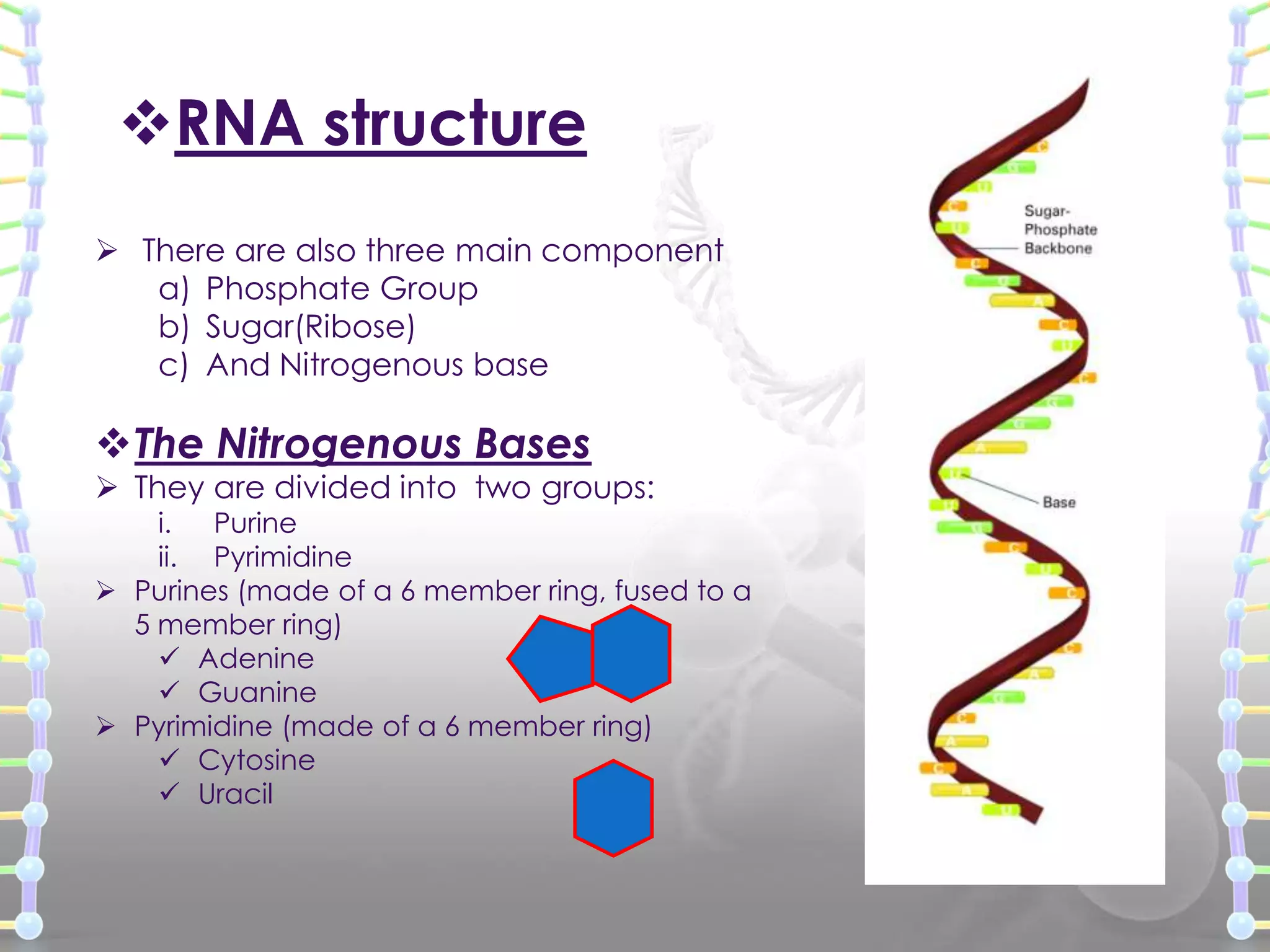
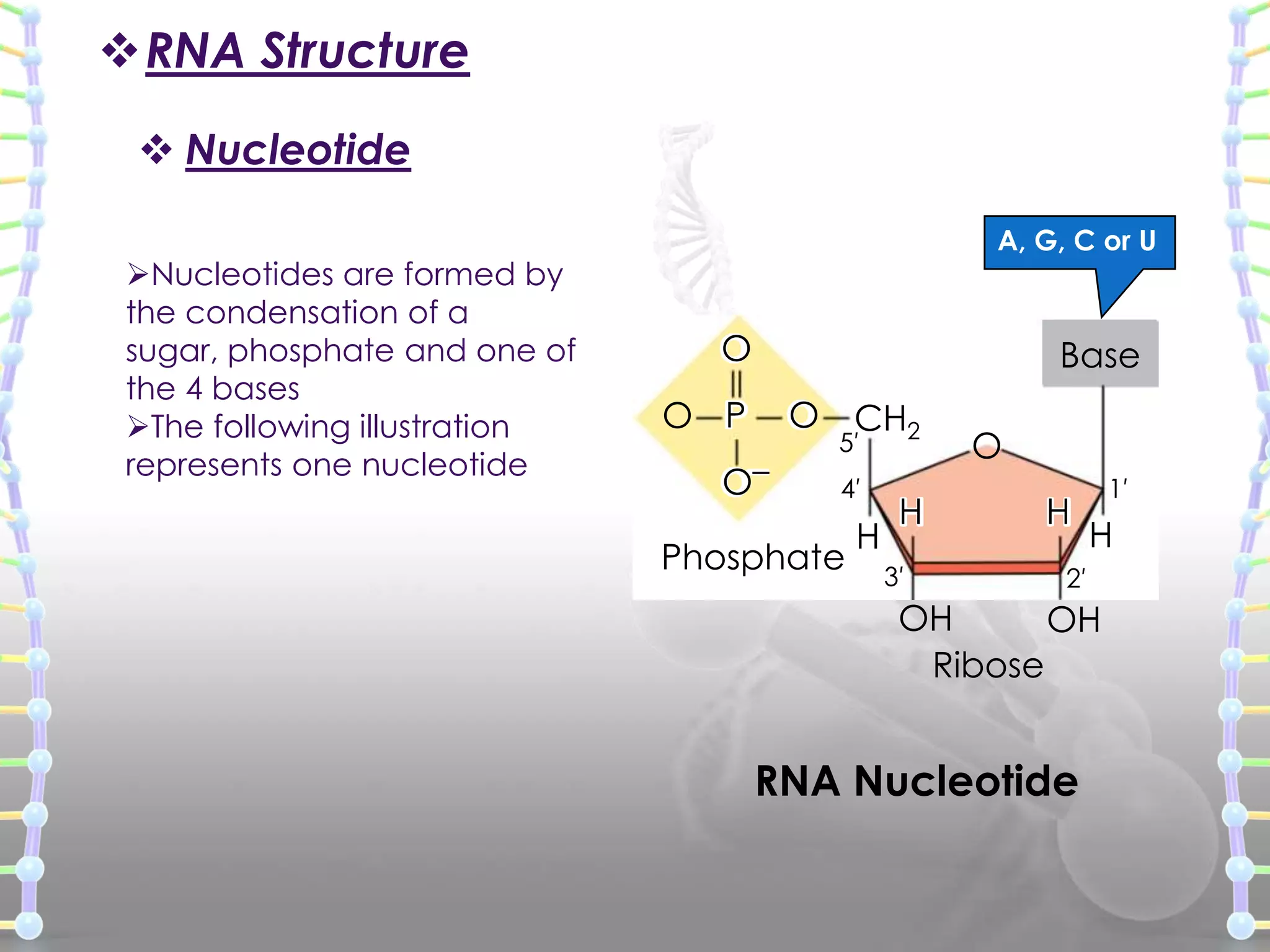
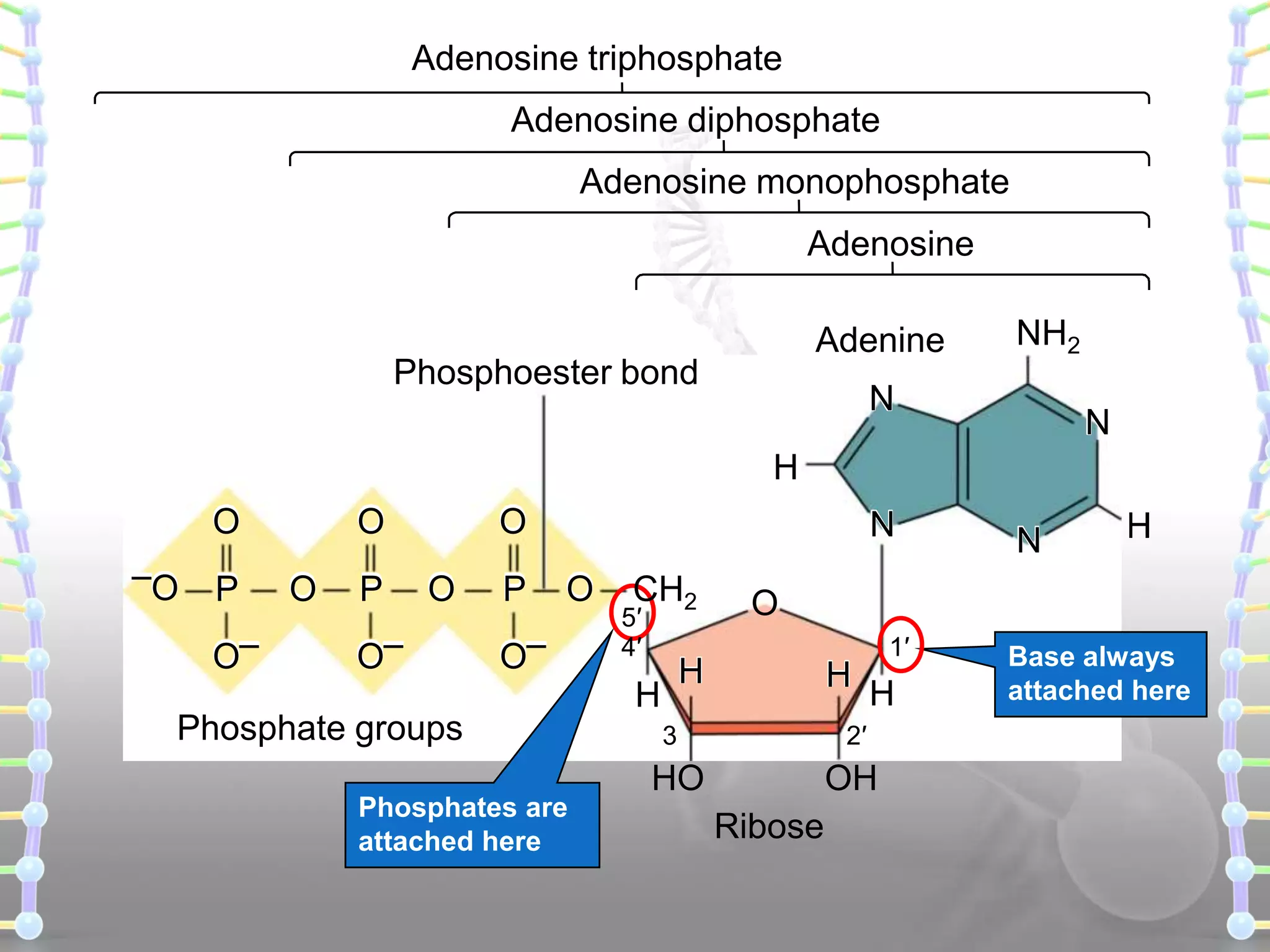
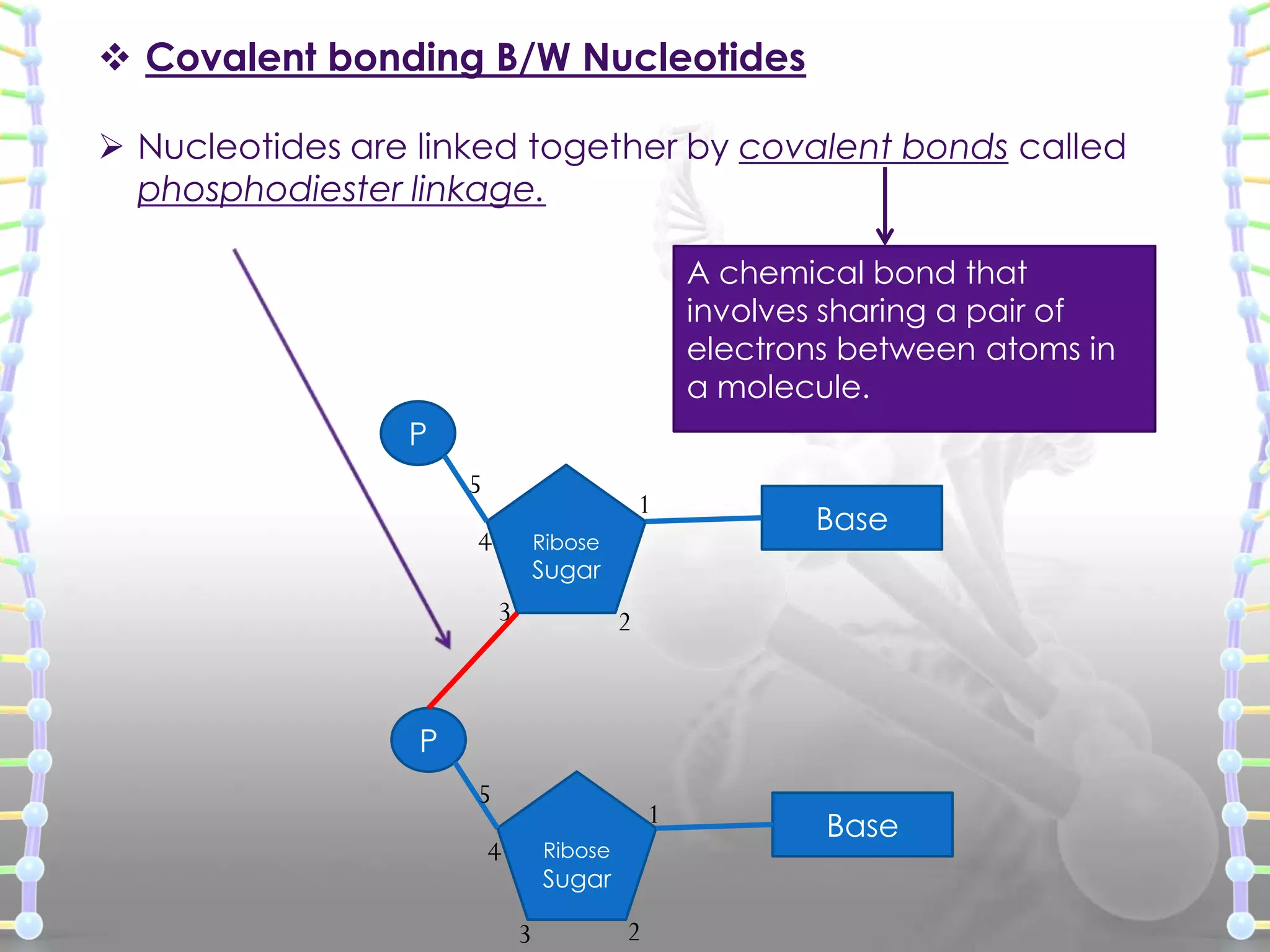
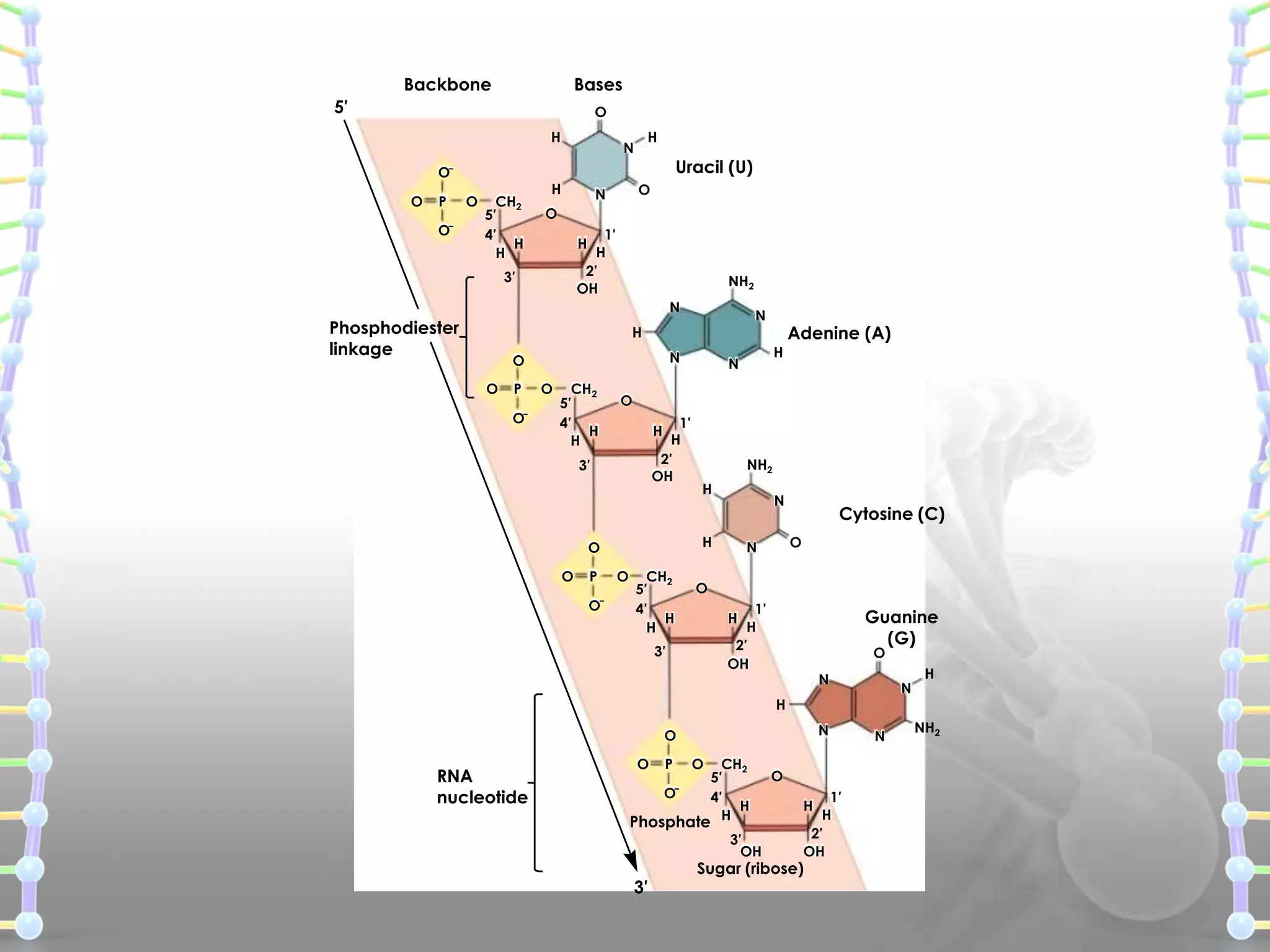
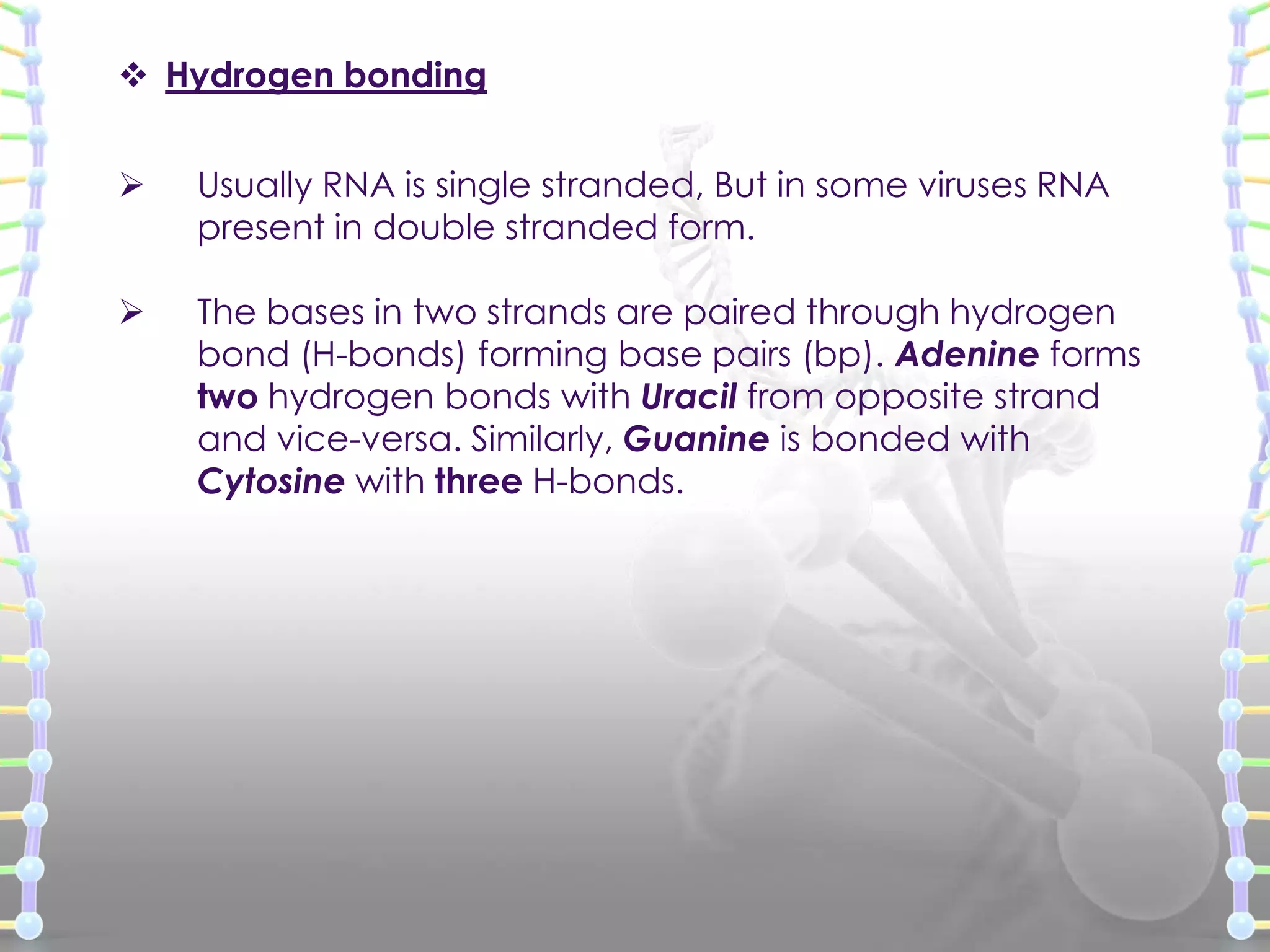
The document provides information on the structure of DNA and RNA. It discusses how DNA was discovered to have a double helix structure by Watson and Crick in 1953 based on prior work by scientists like Franklin, Wilkins, Chargaff and Pauling. It describes the key components of DNA including the sugar-phosphate backbone, nitrogenous bases, and how the bases pair up in the double helix structure. It also discusses different DNA structures like A, B and Z-DNA and how DNA packages into nucleosomes and chromosomes. For RNA, it notes that it is similar to DNA but contains the sugar ribose and base uracil instead of thymine.














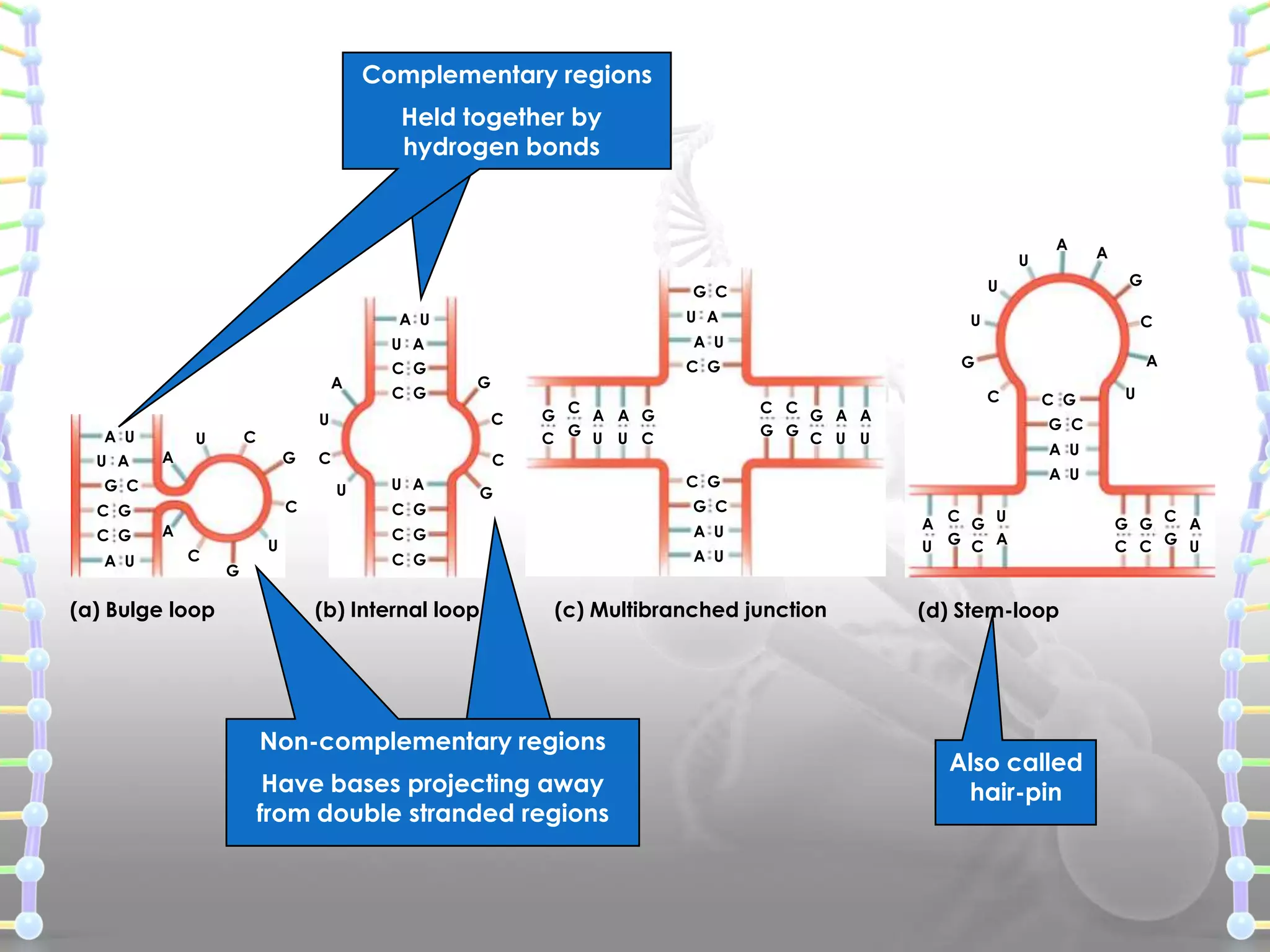
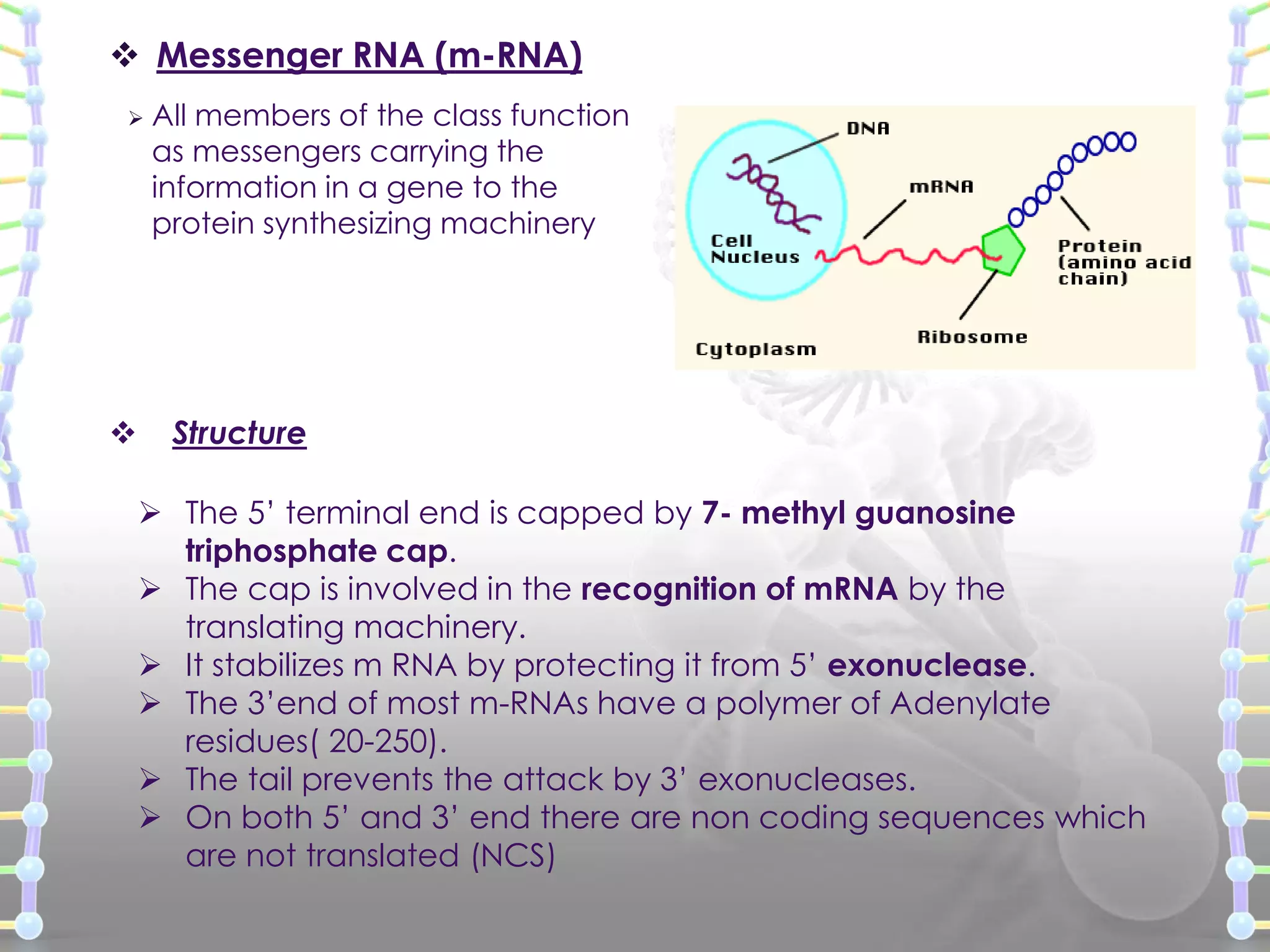
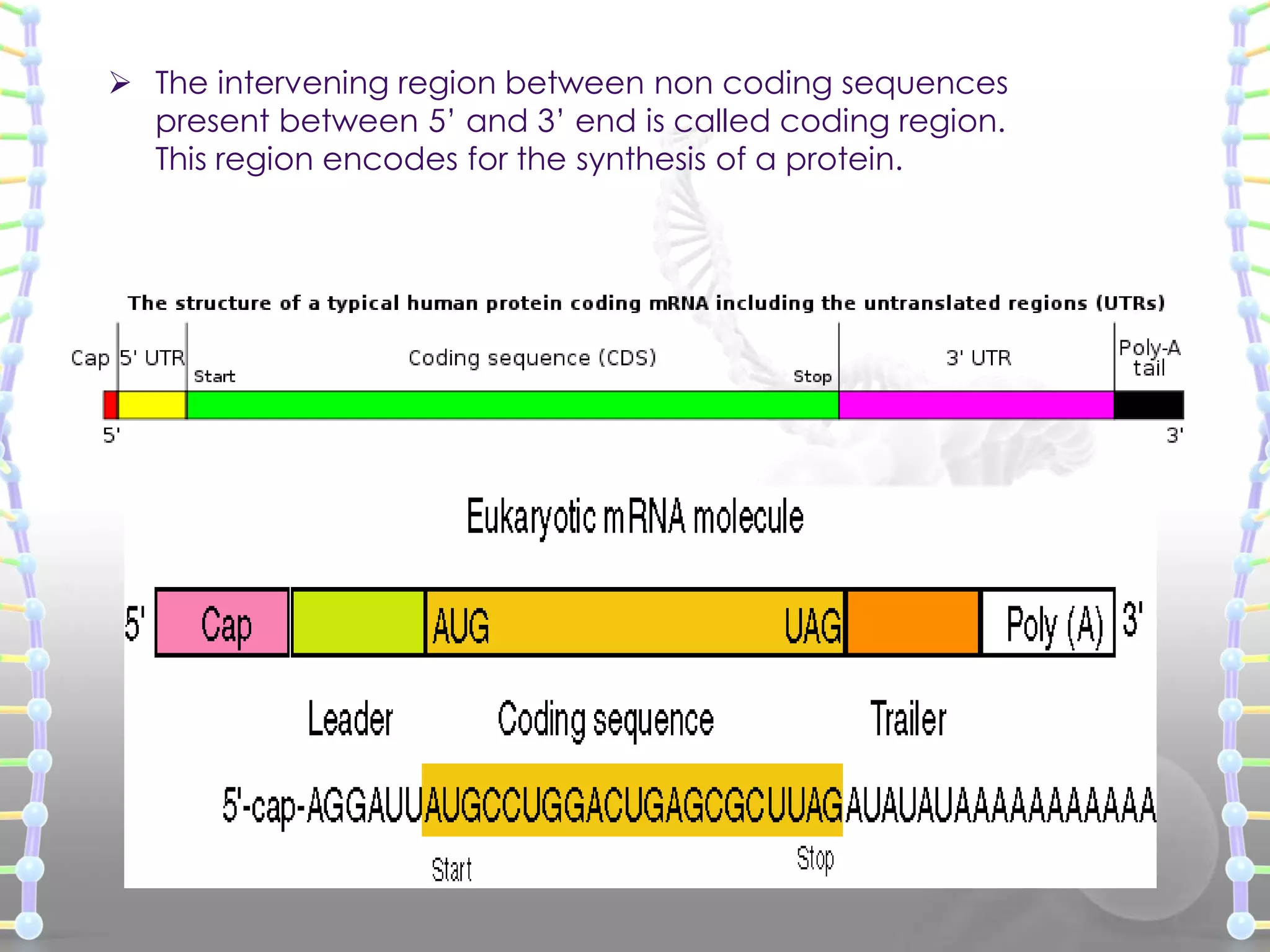
![ Heterogeneous nuclear RNA (hnRNA) [Precursor mRNA]
In mammalian nuclei , hnRNA is the immediate
product of gene transcription
The nuclear product is heterogeneous in size
(Variable) and is very large.
75 % of hnRNA is degraded in the nucleus,
only 25% is processed to mature m RNA.
Mature m –RNA is formed from primary transcript
by capping, tailing, splicing and base modification.](https://image.slidesharecdn.com/structureofdnarna-140209131401-phpapp01/75/Structure-of-dna-and-rna-58-2048.jpg)