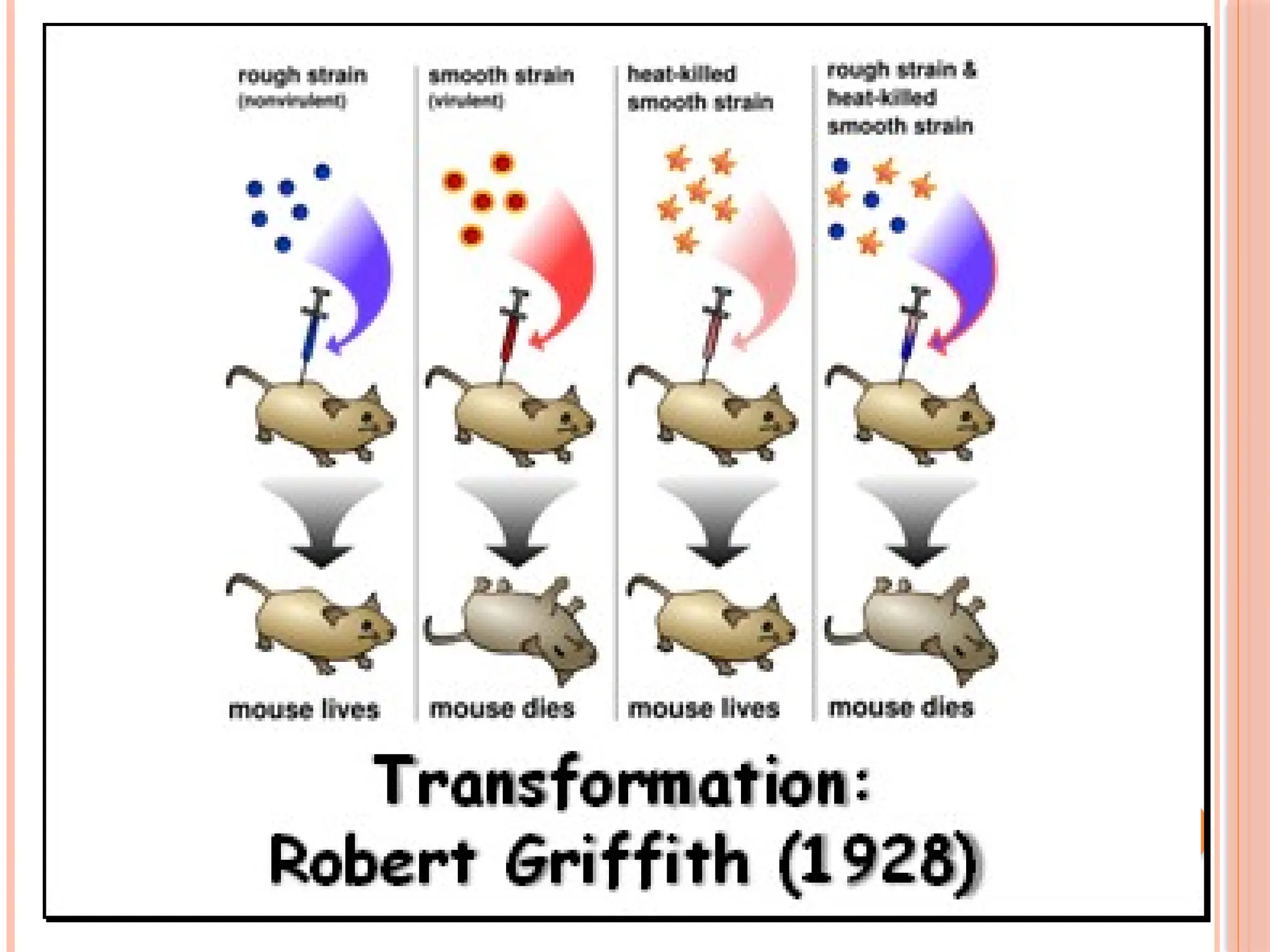
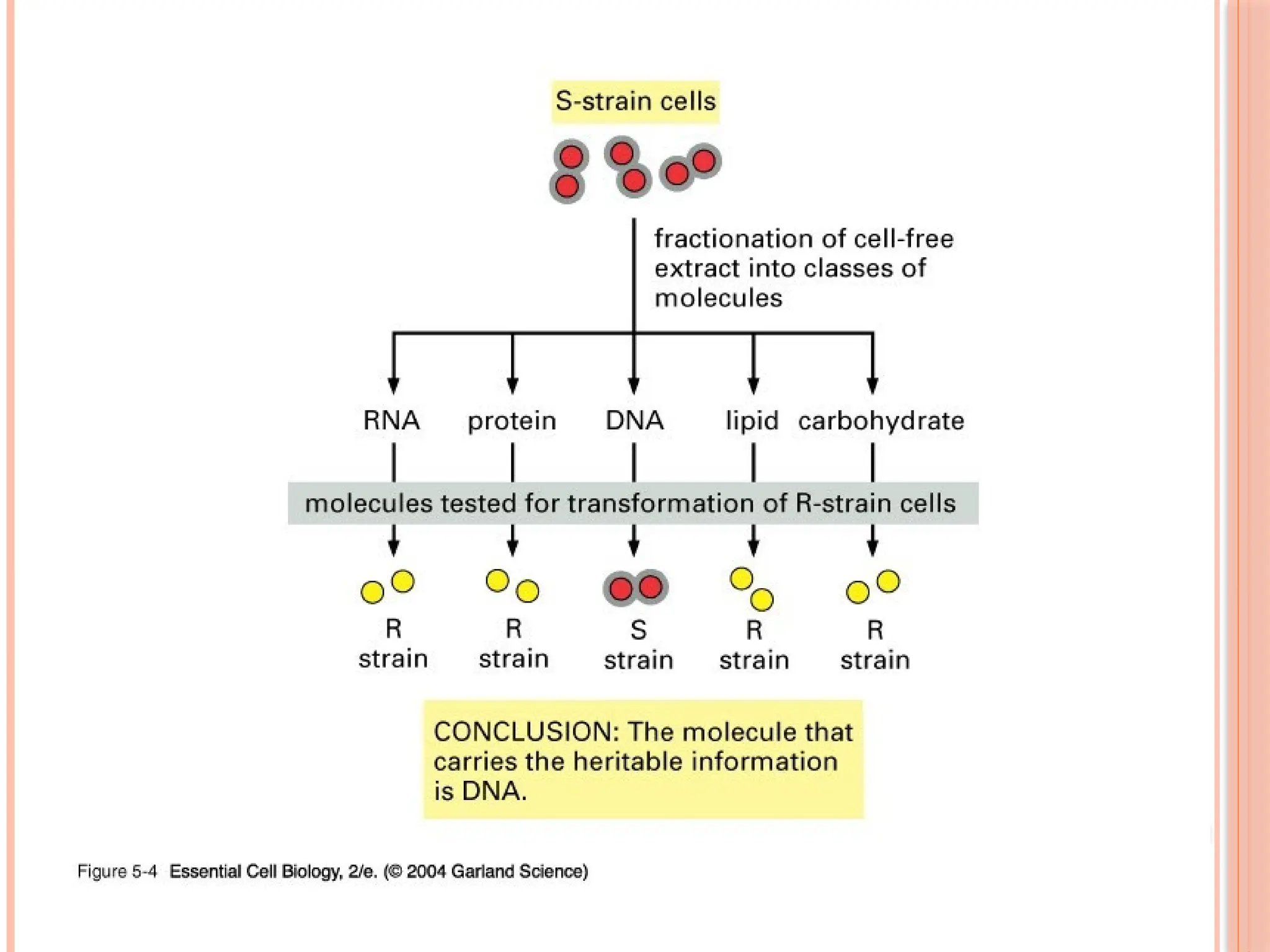
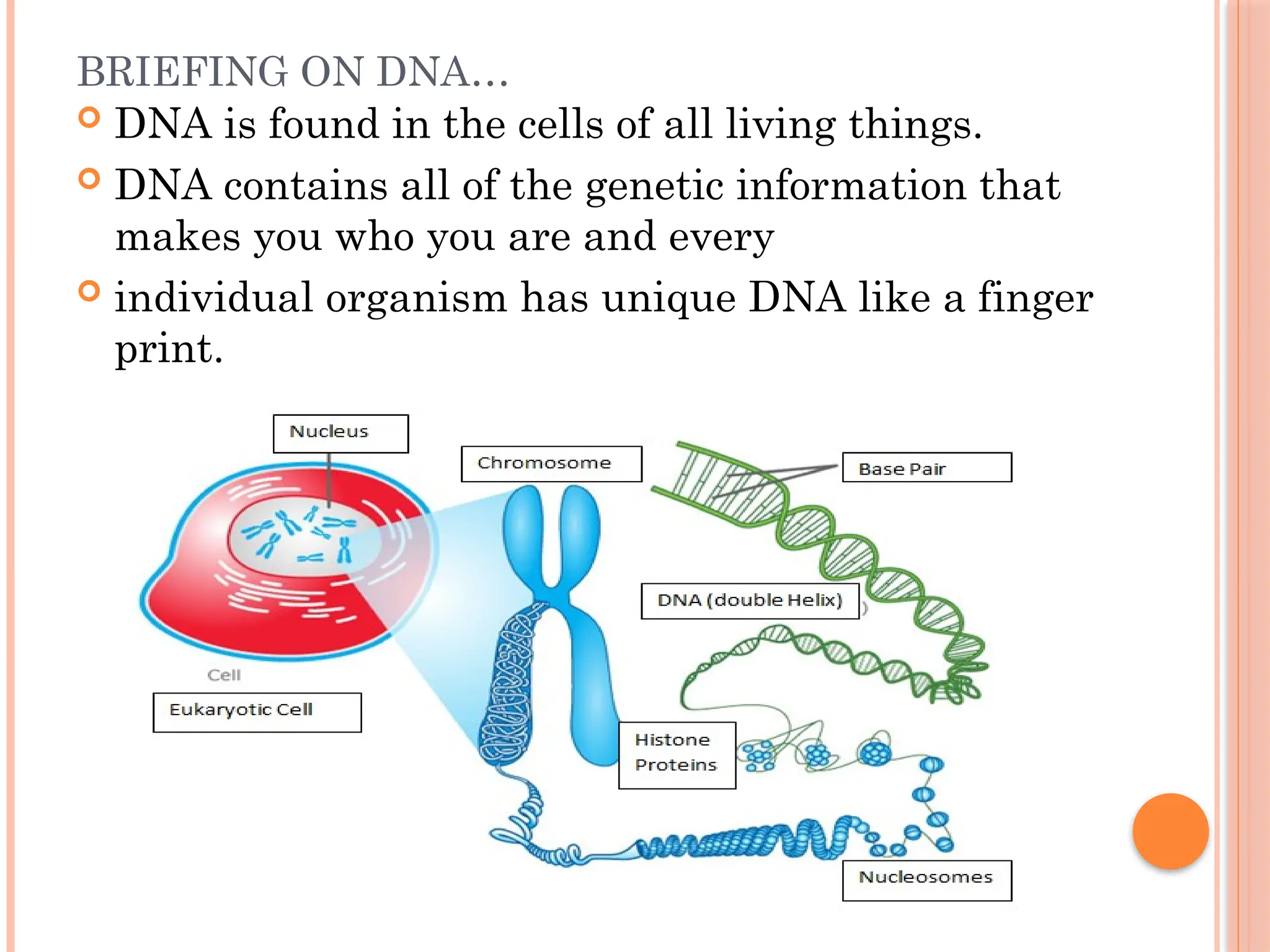
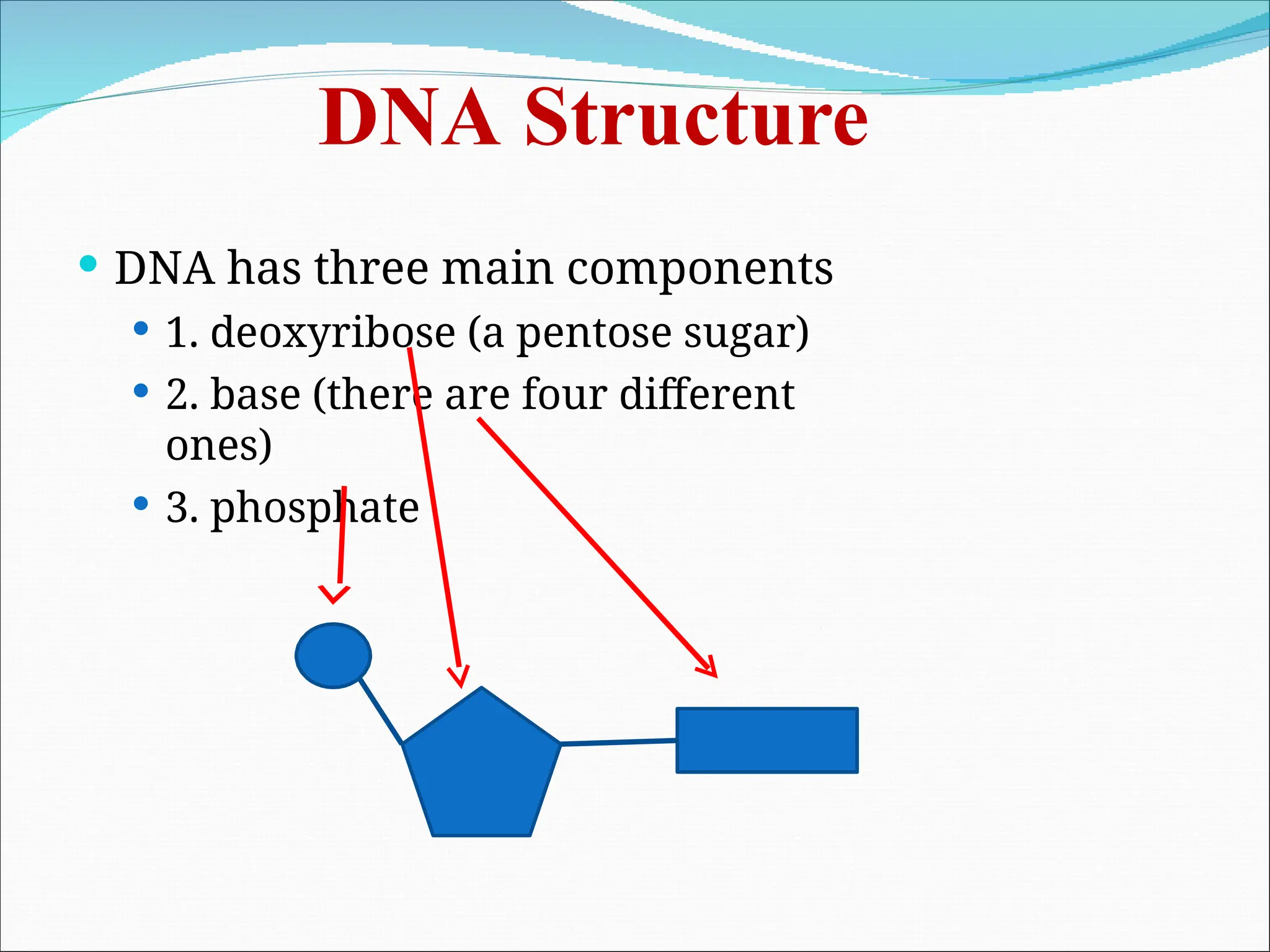
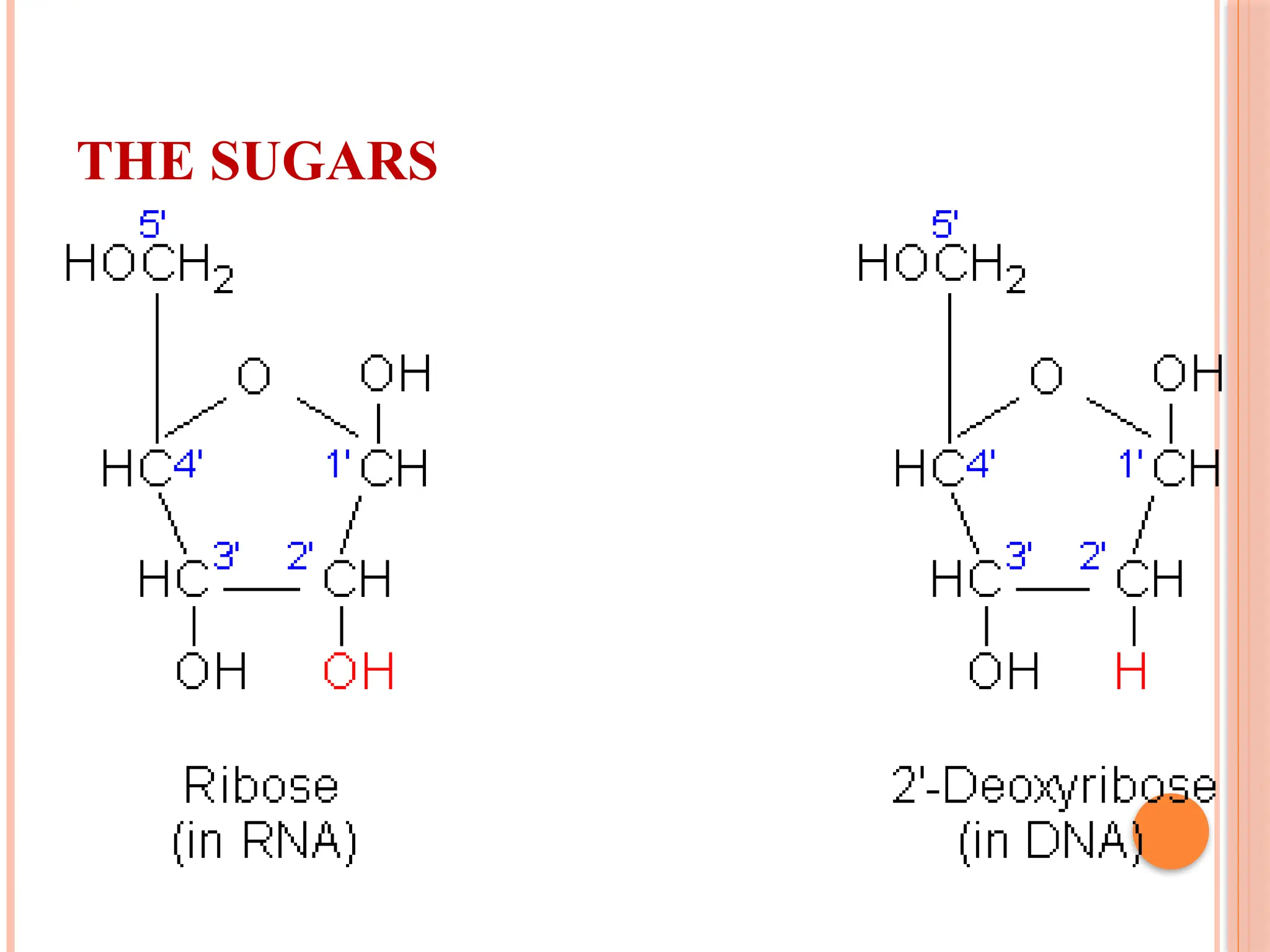
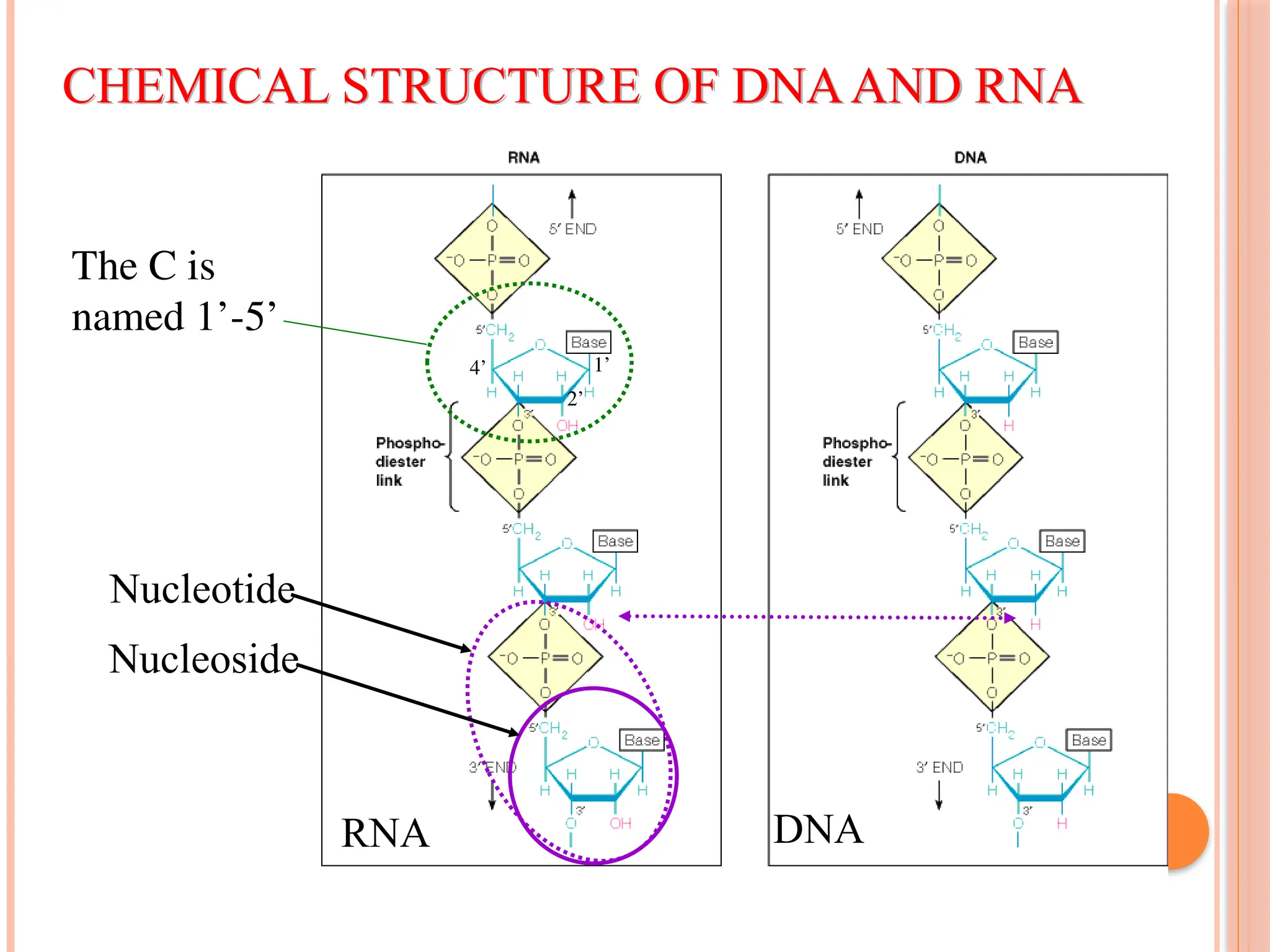
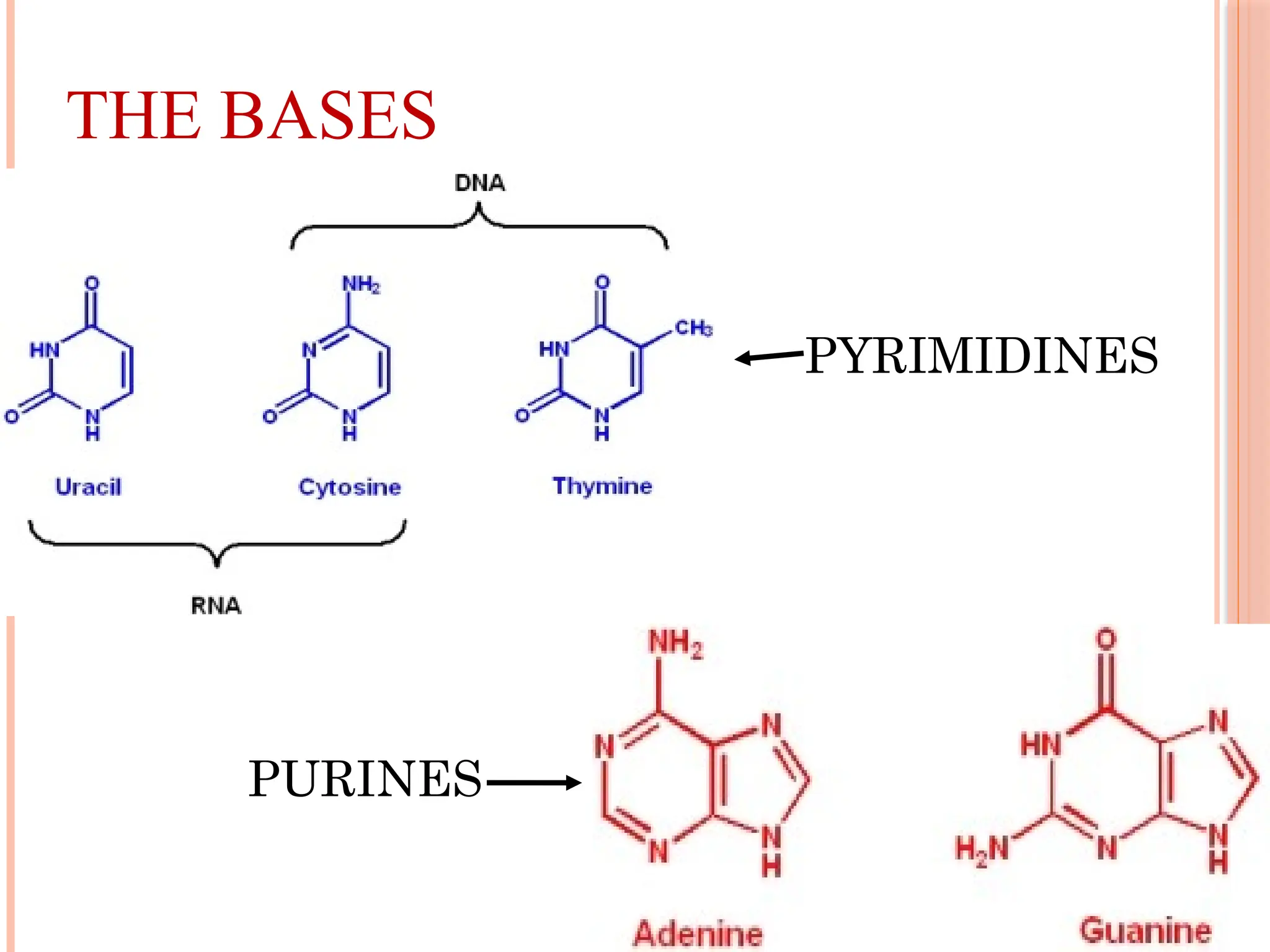
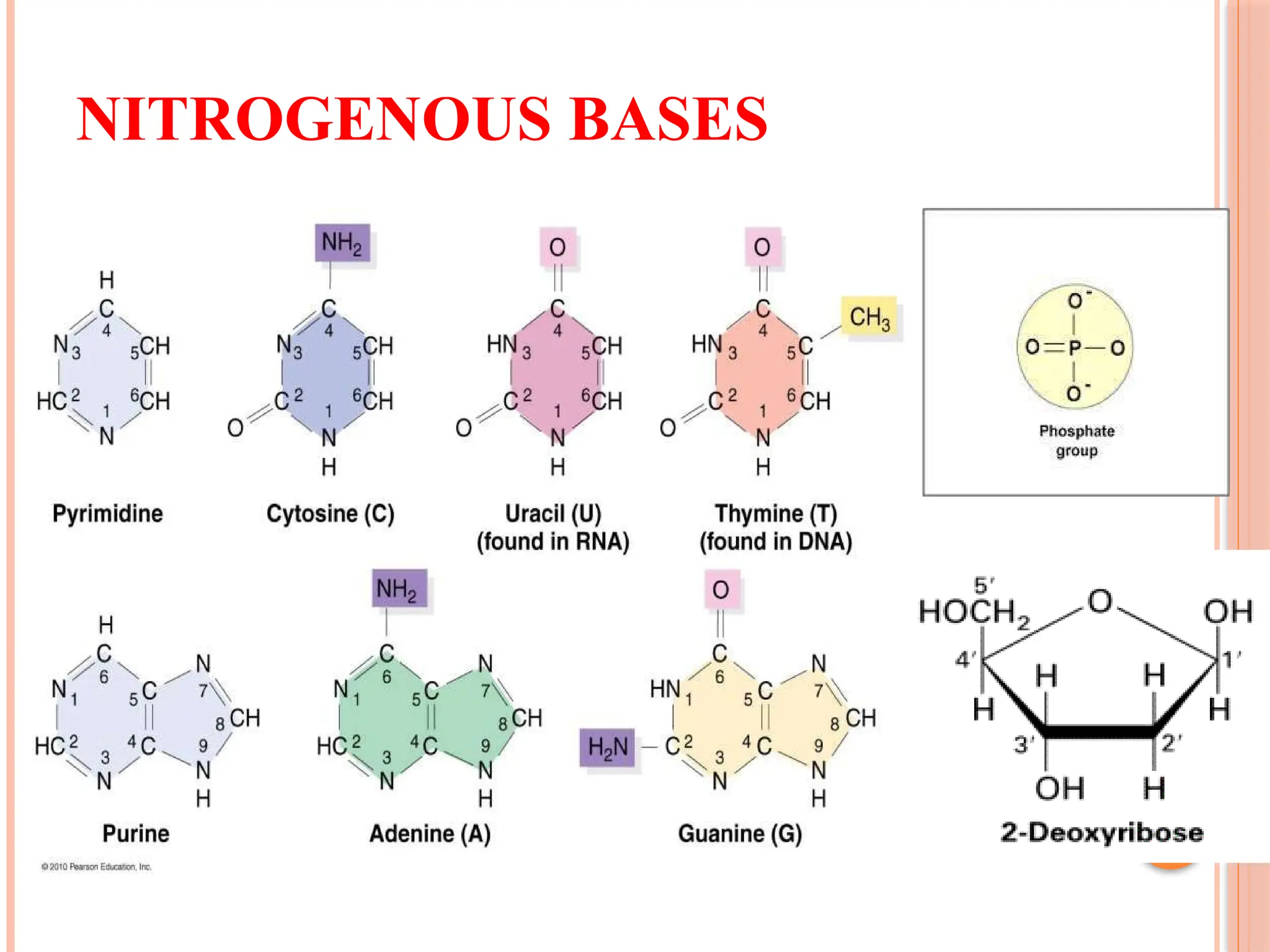
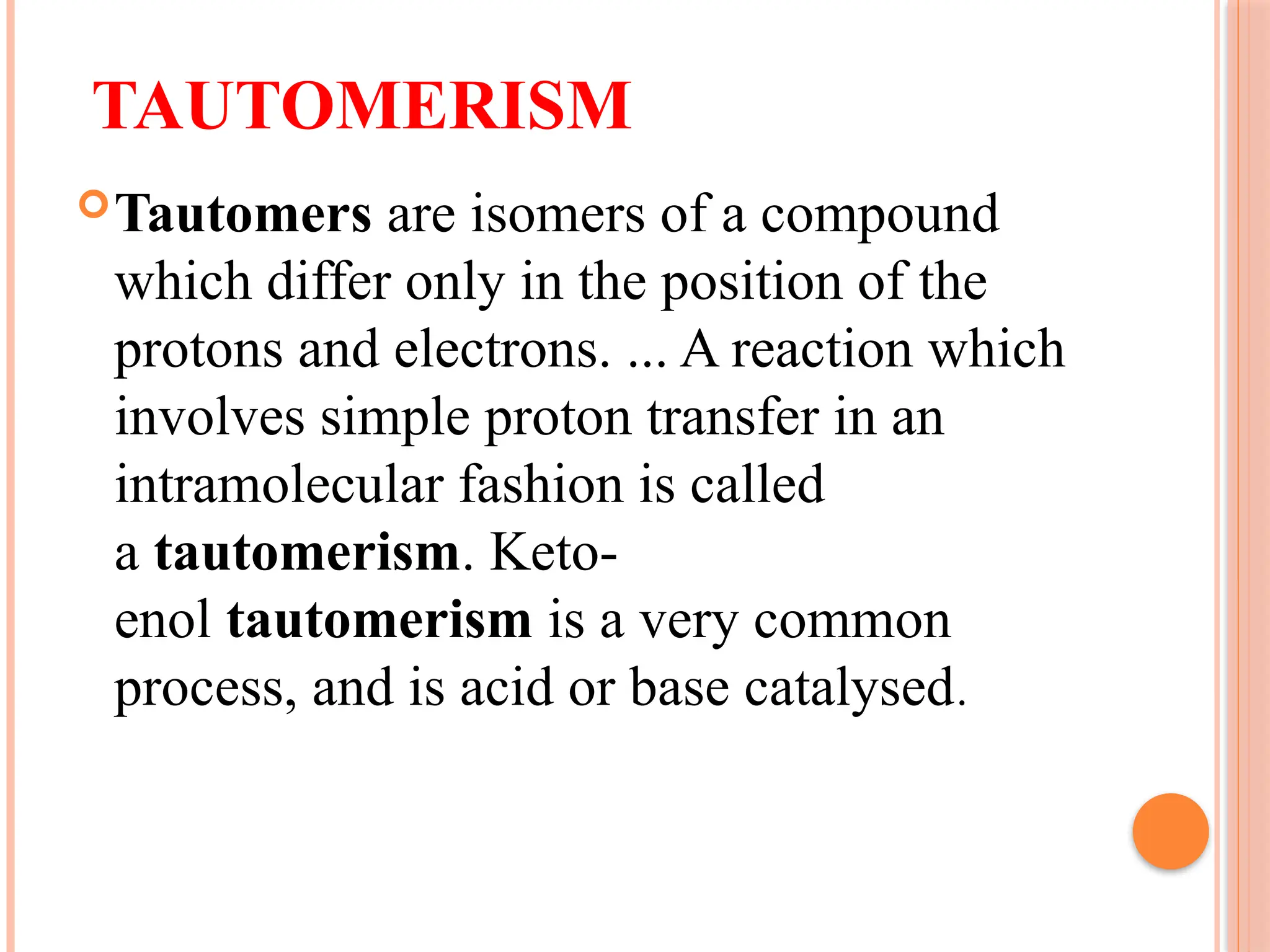
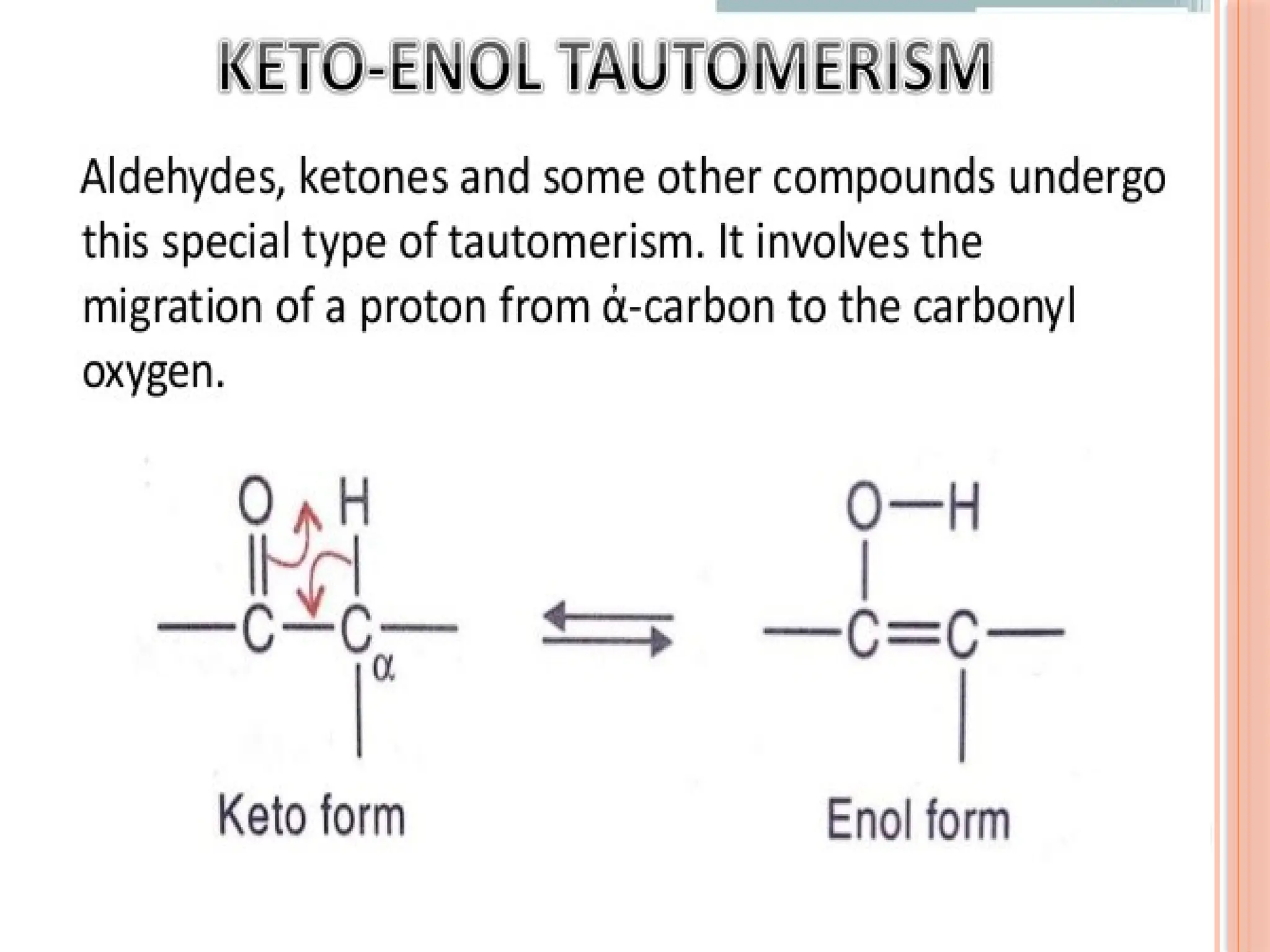
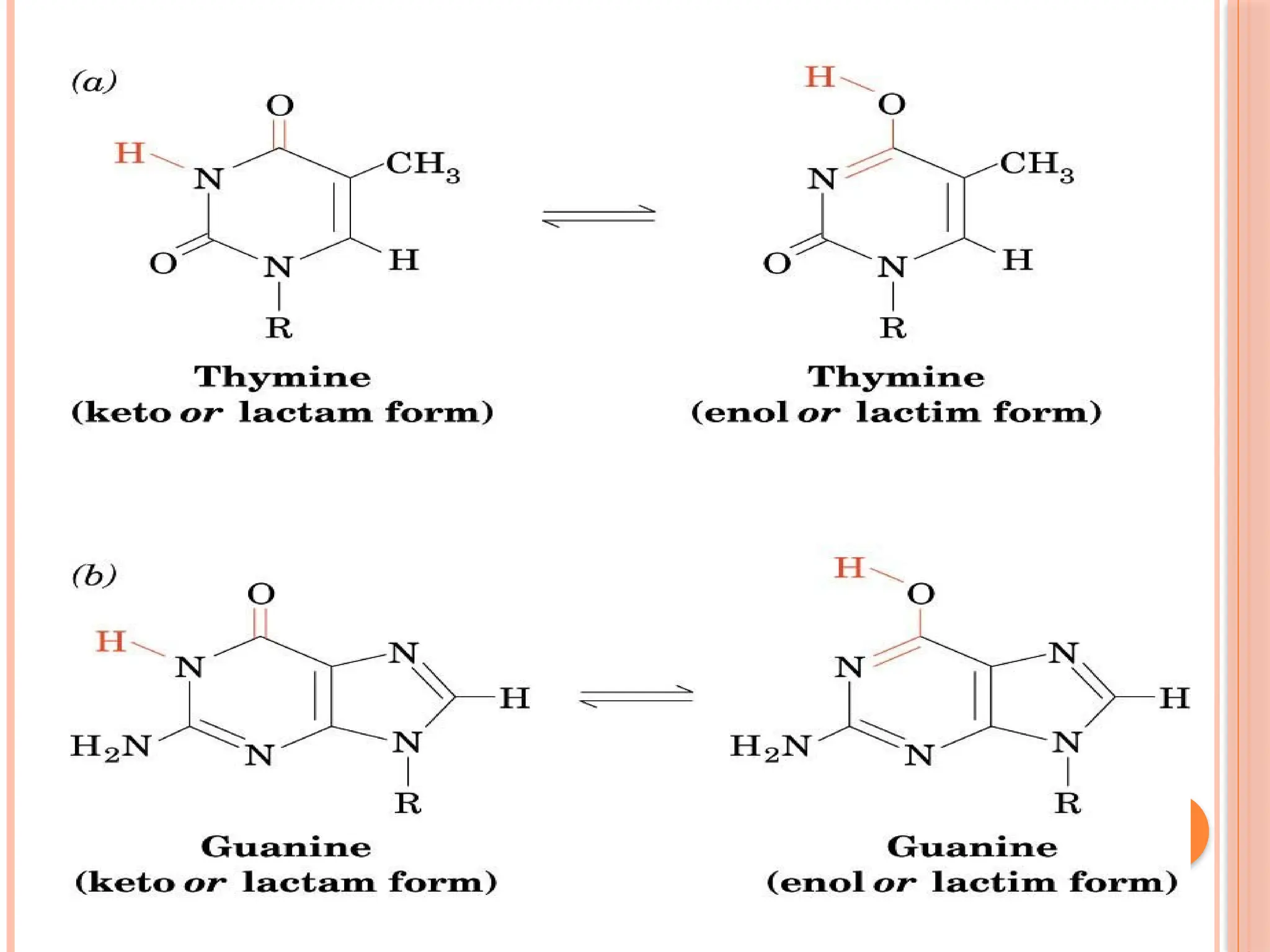
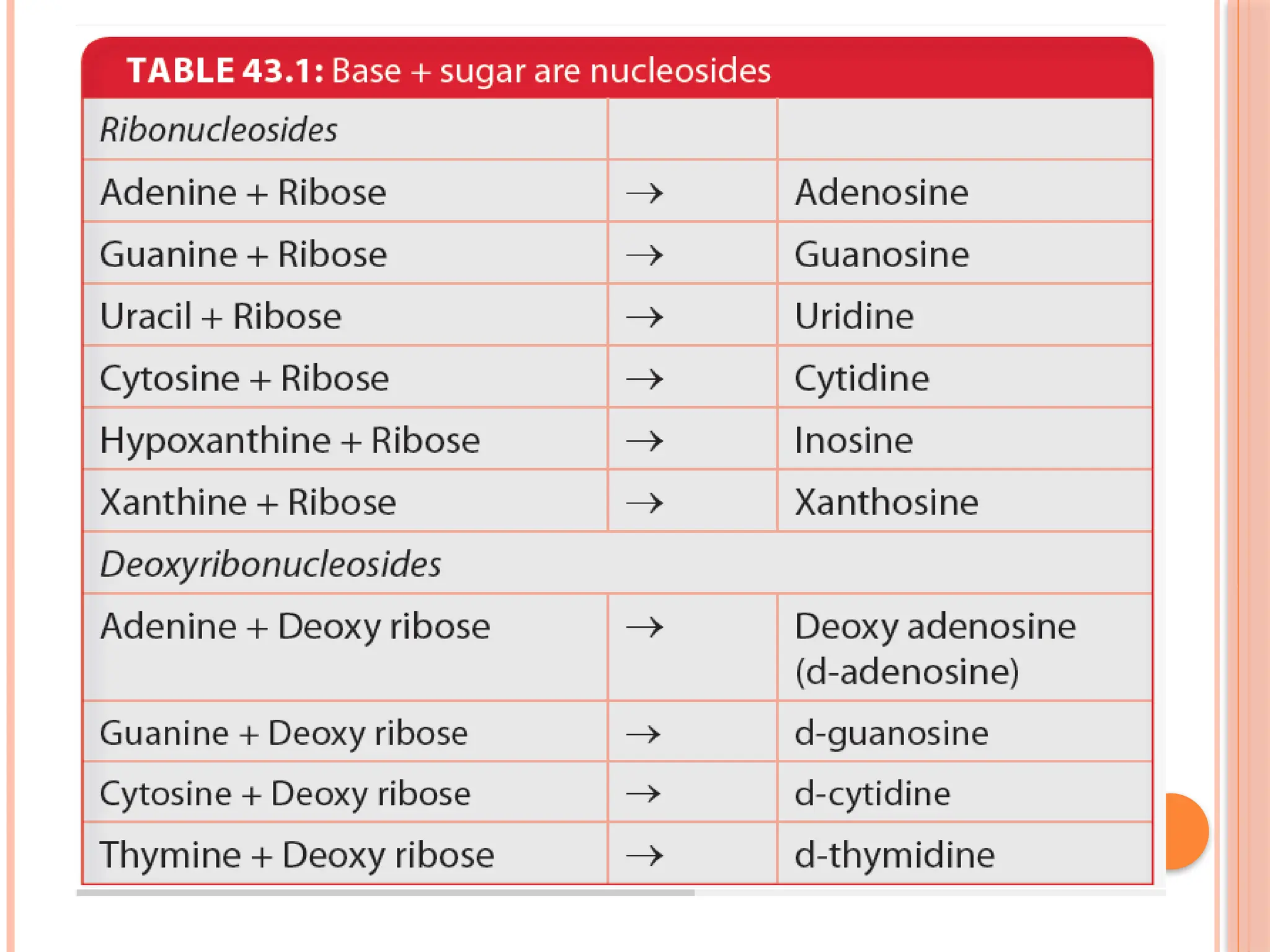
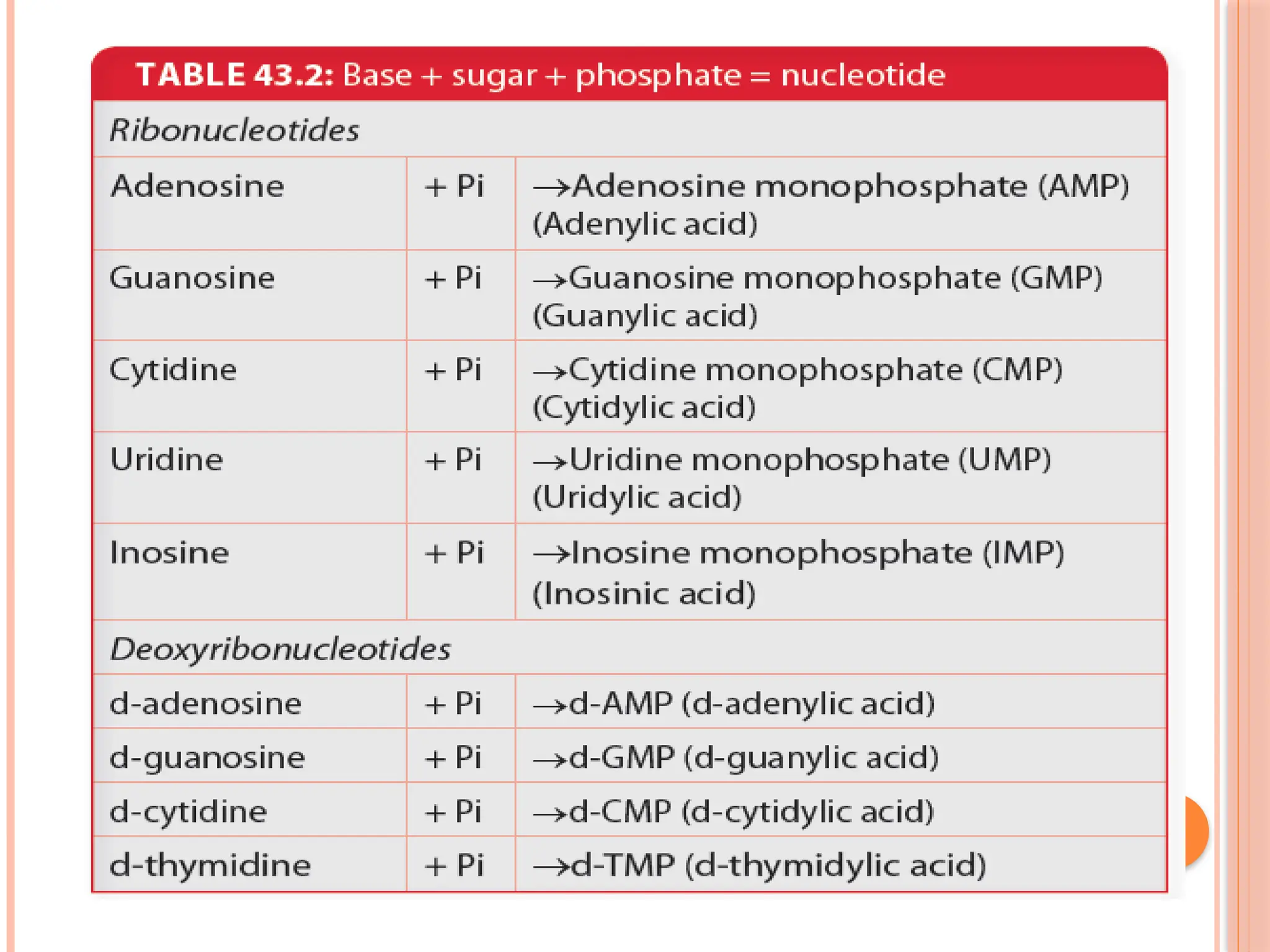
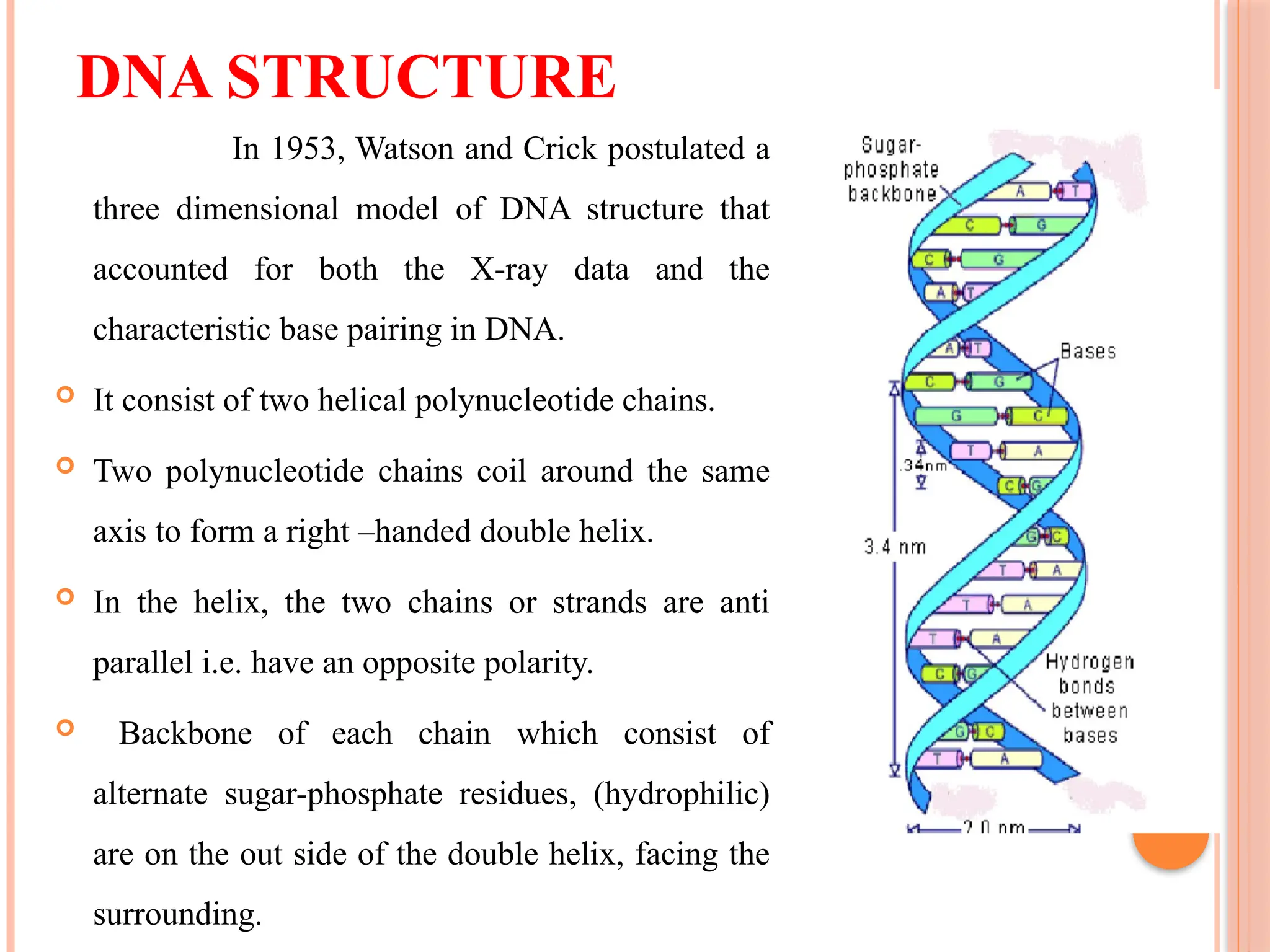
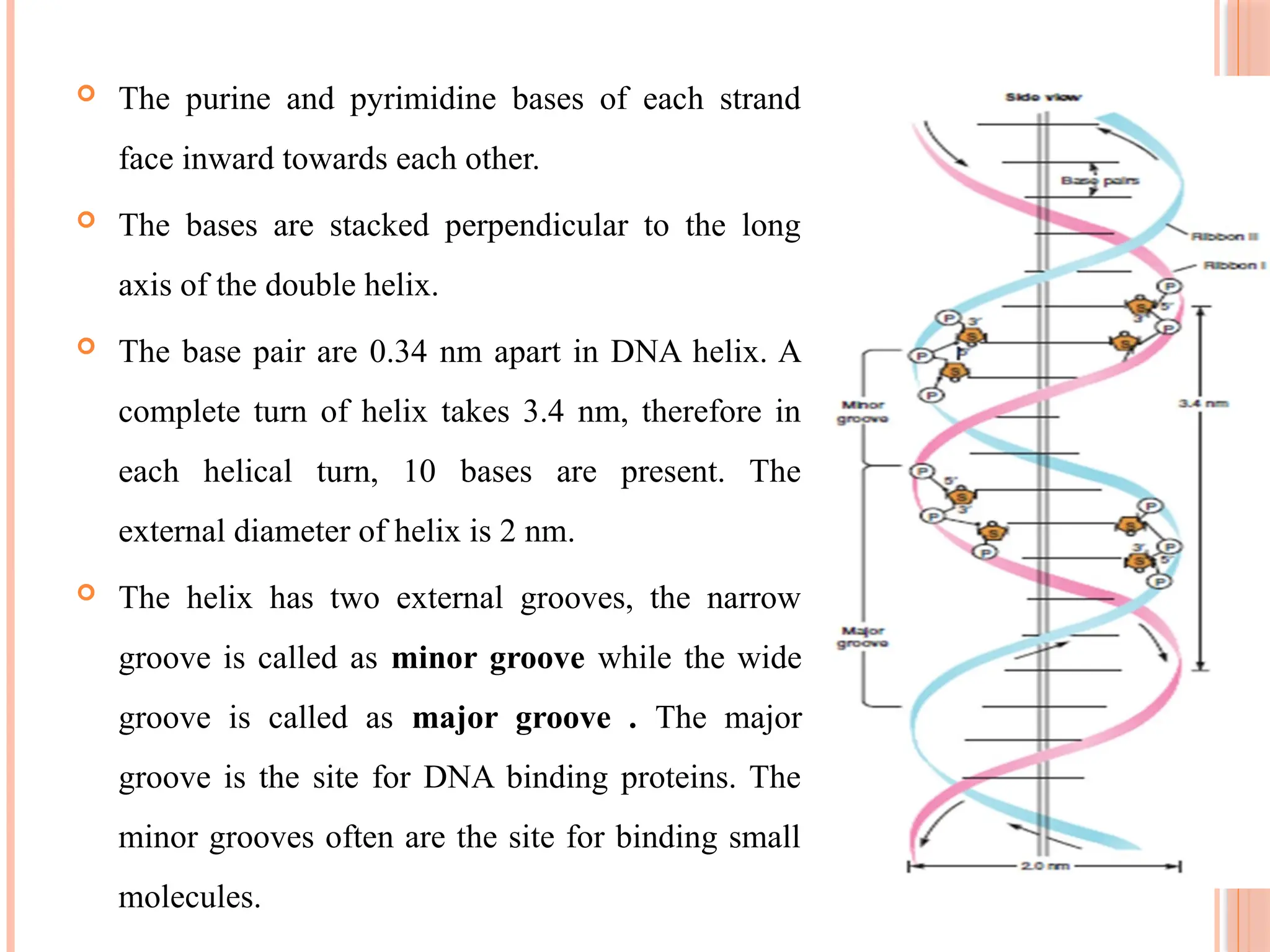
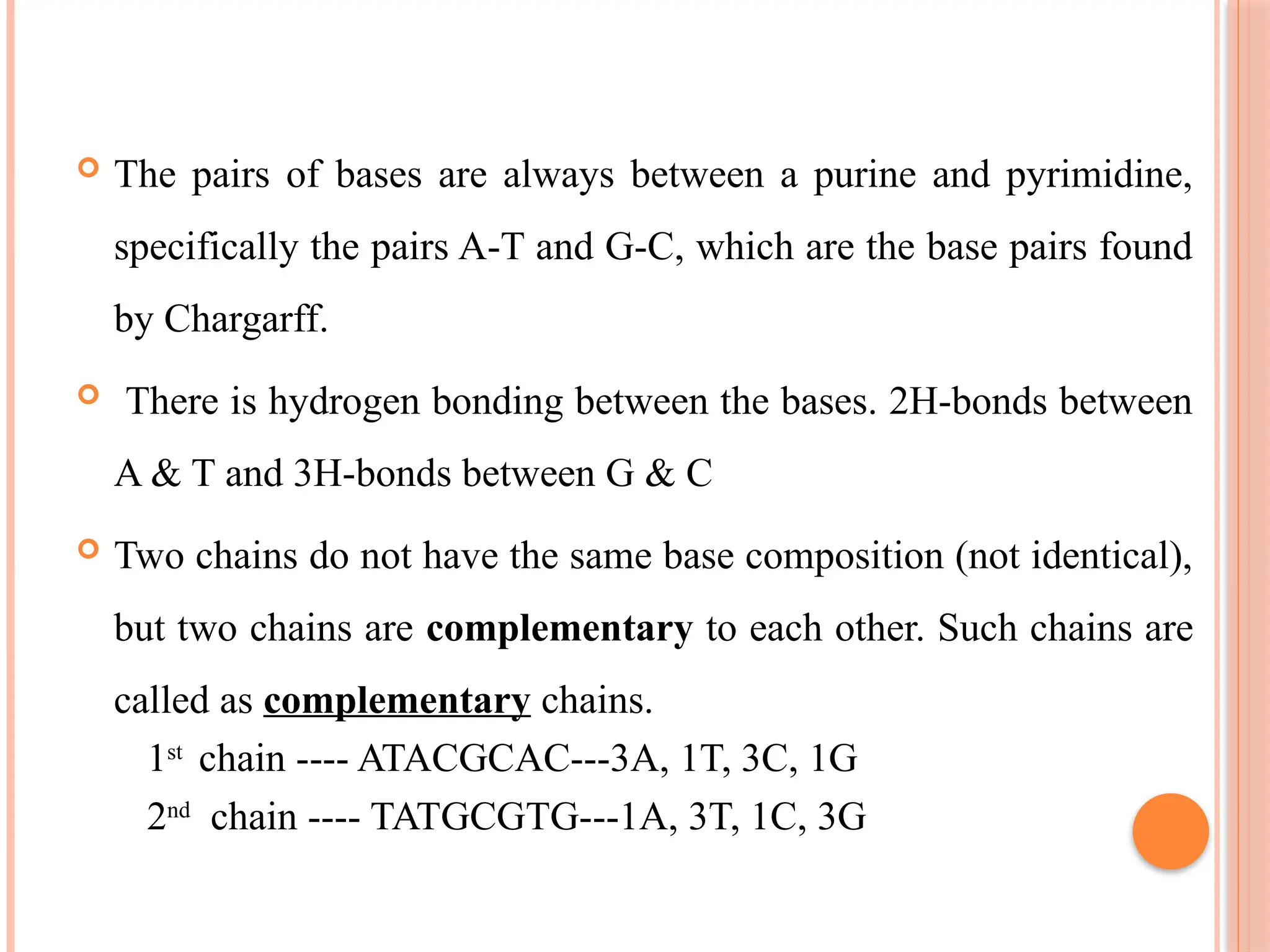
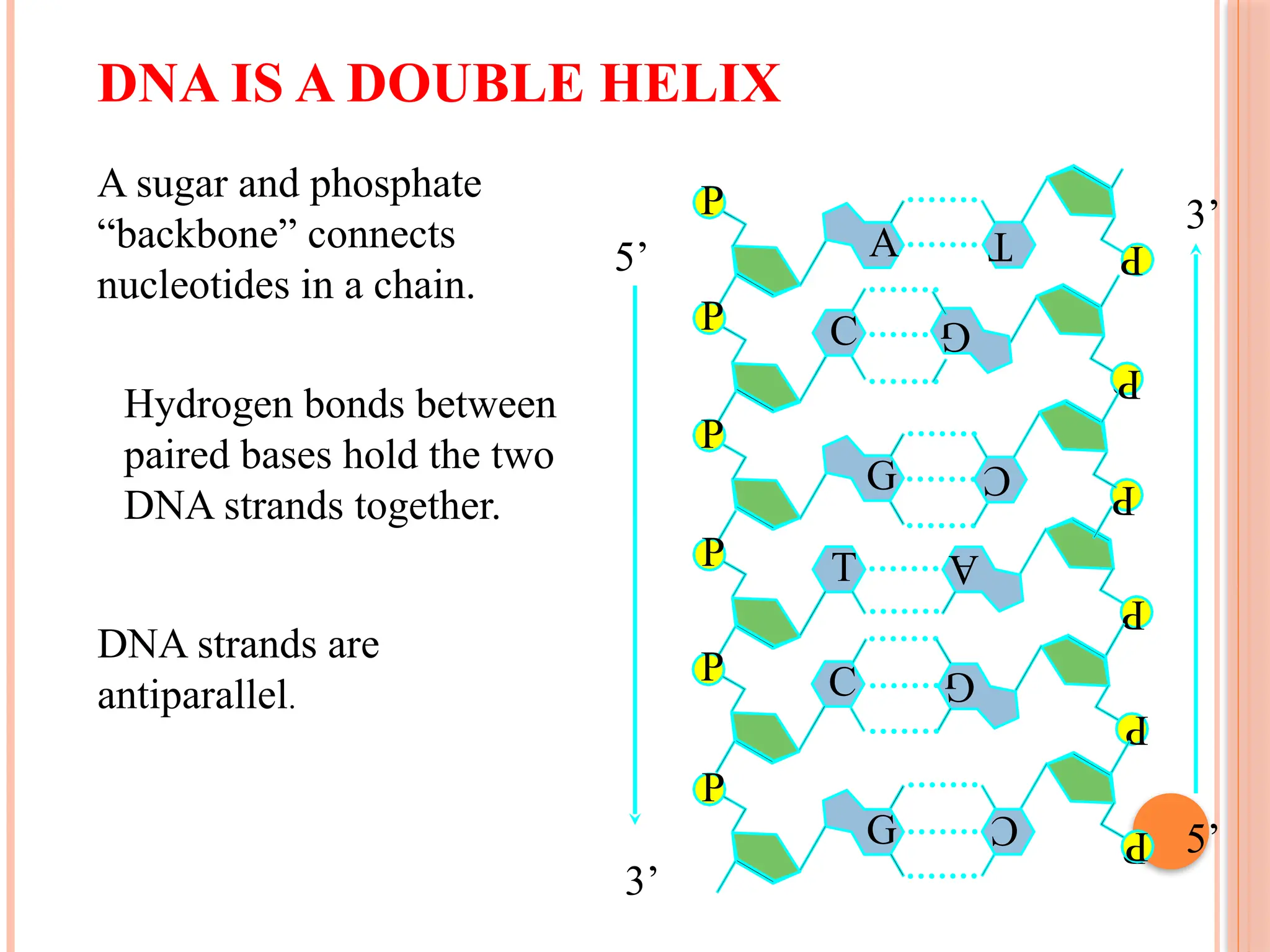
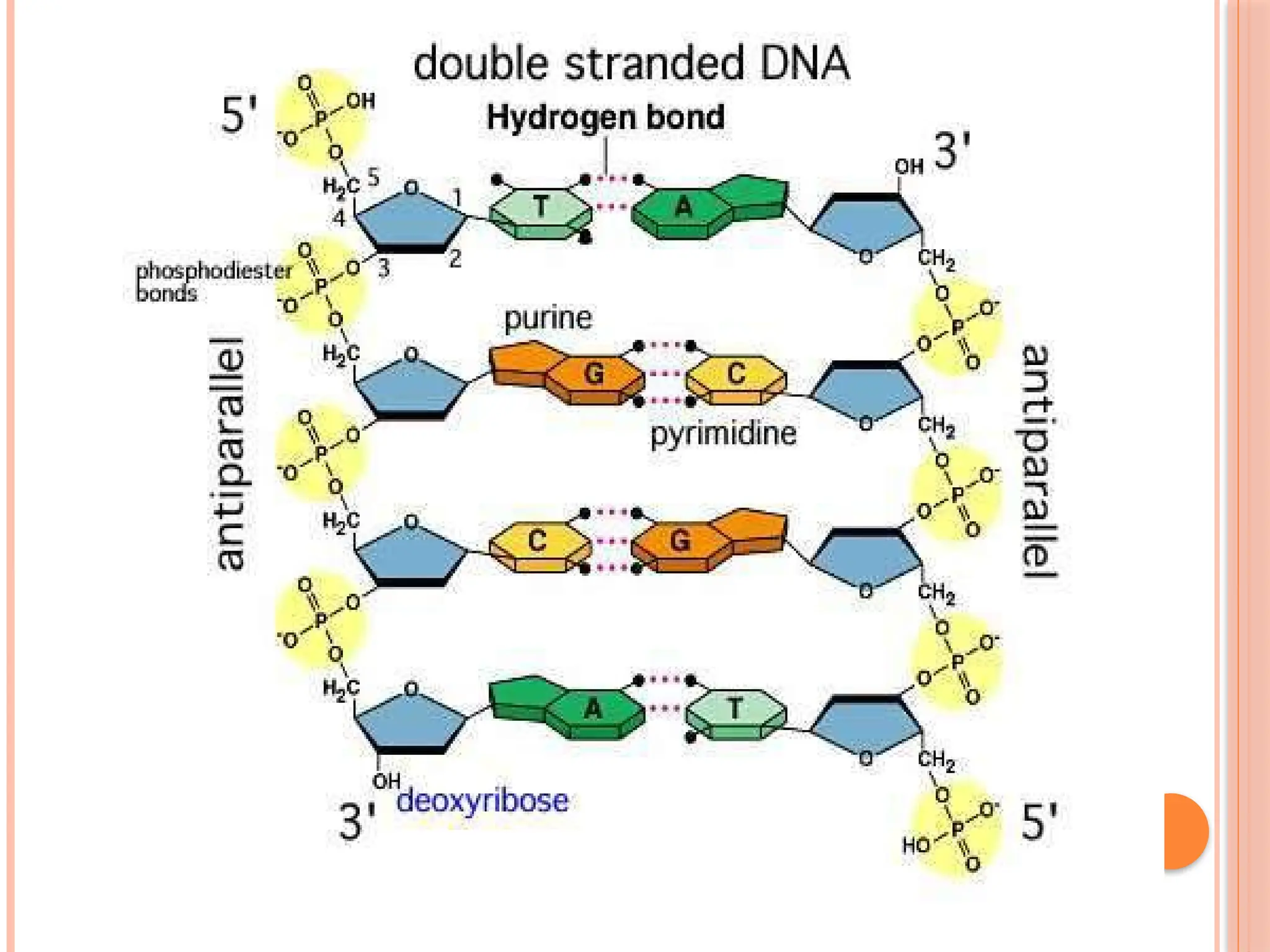
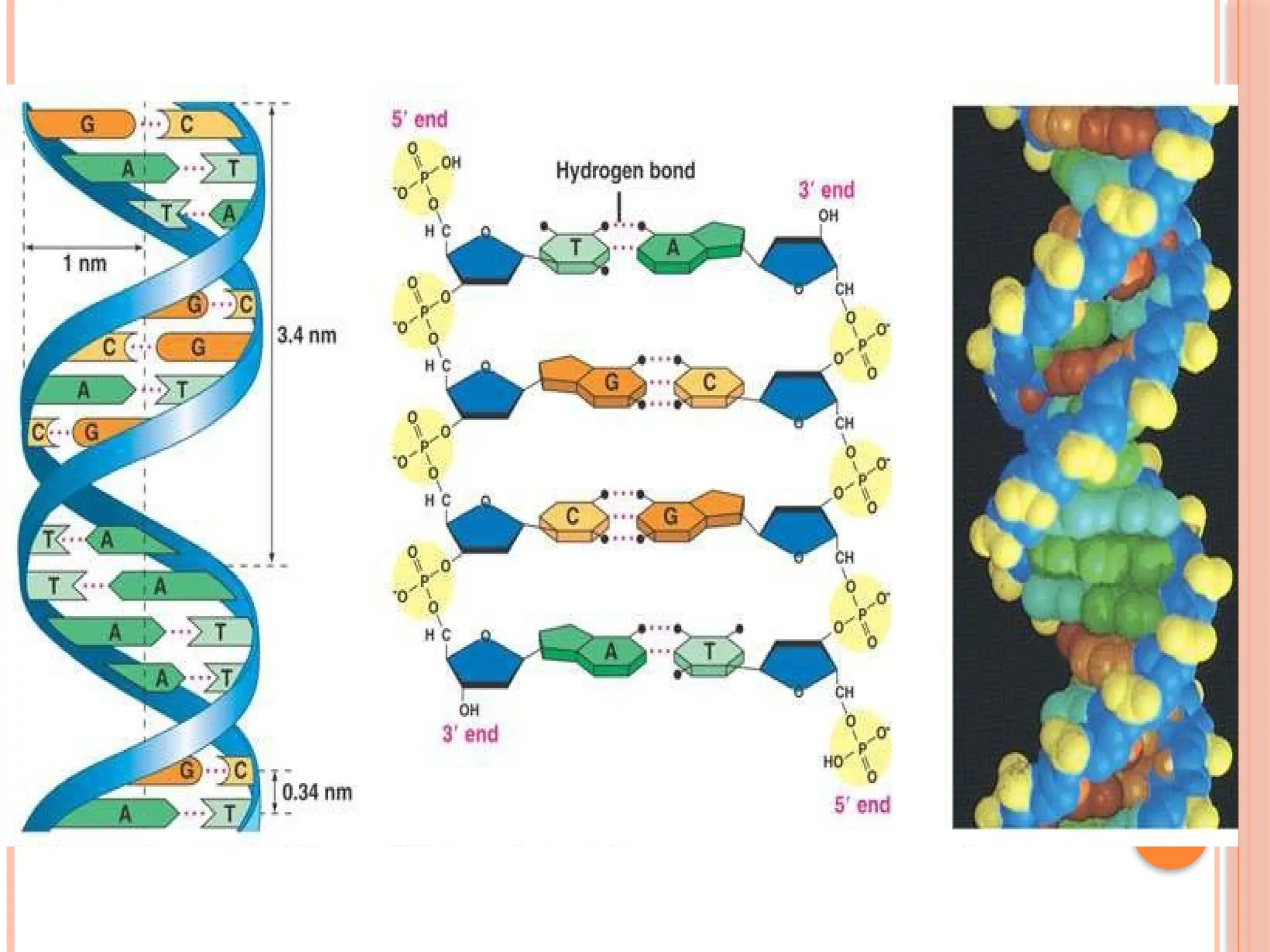
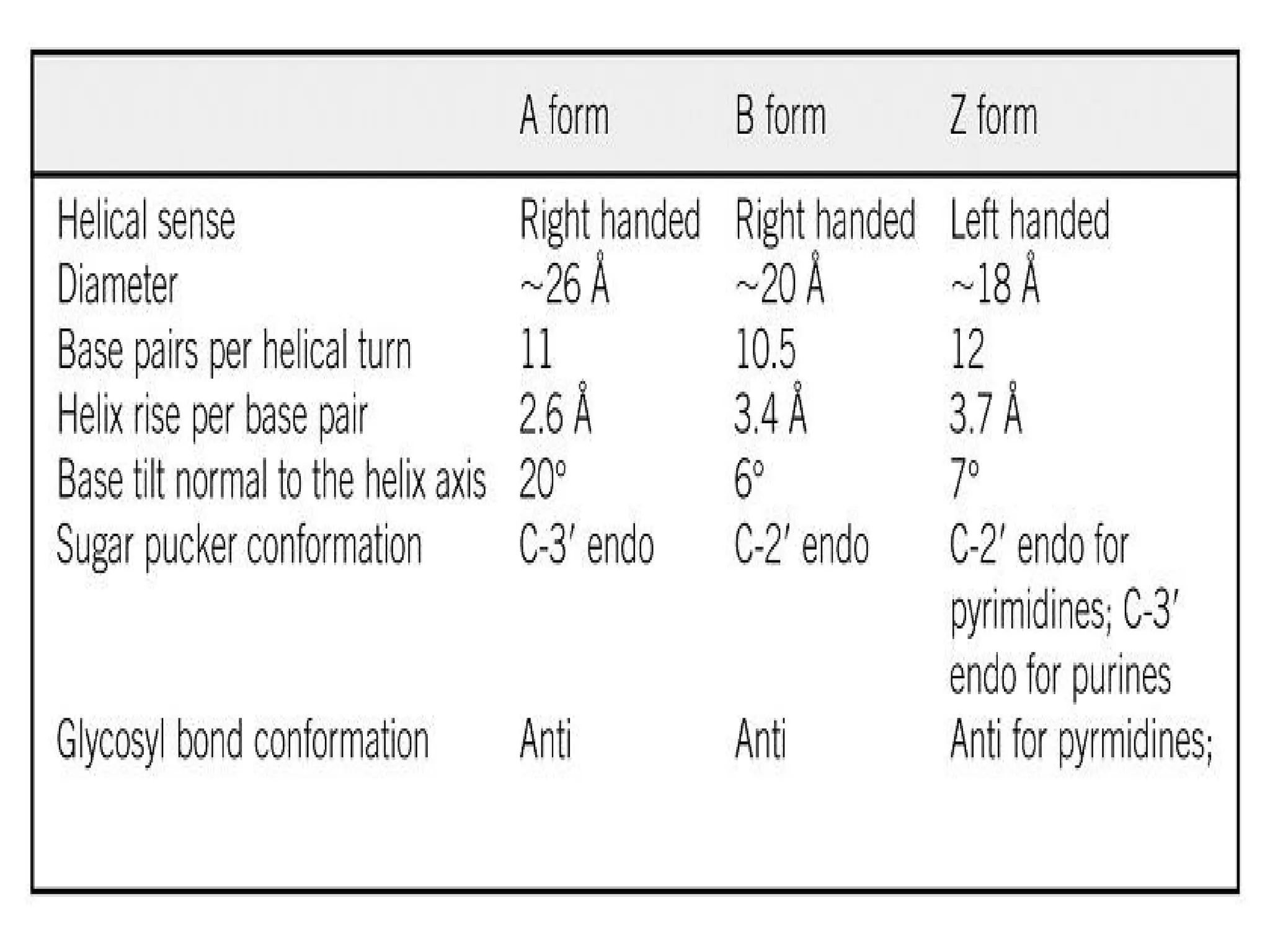
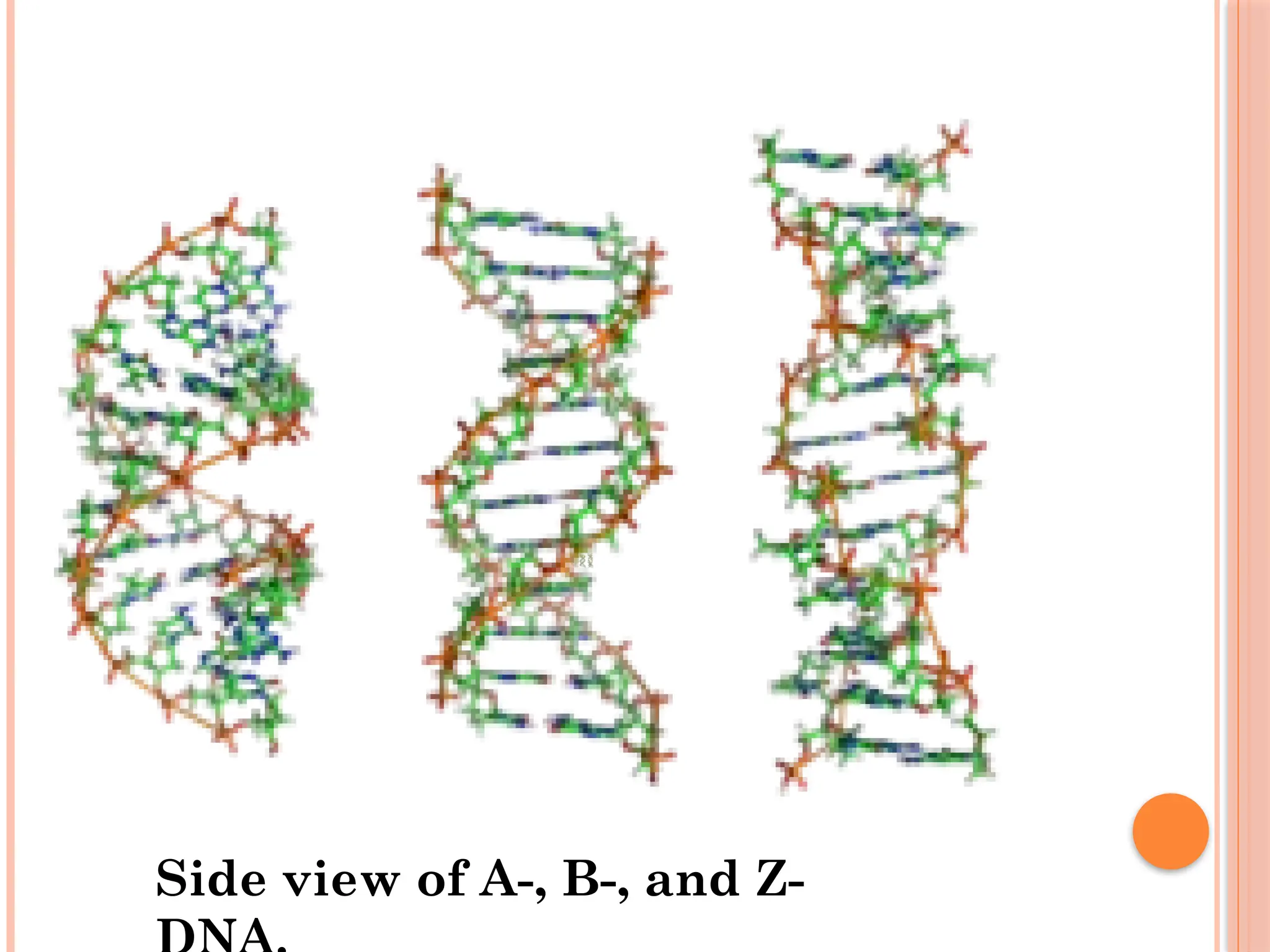
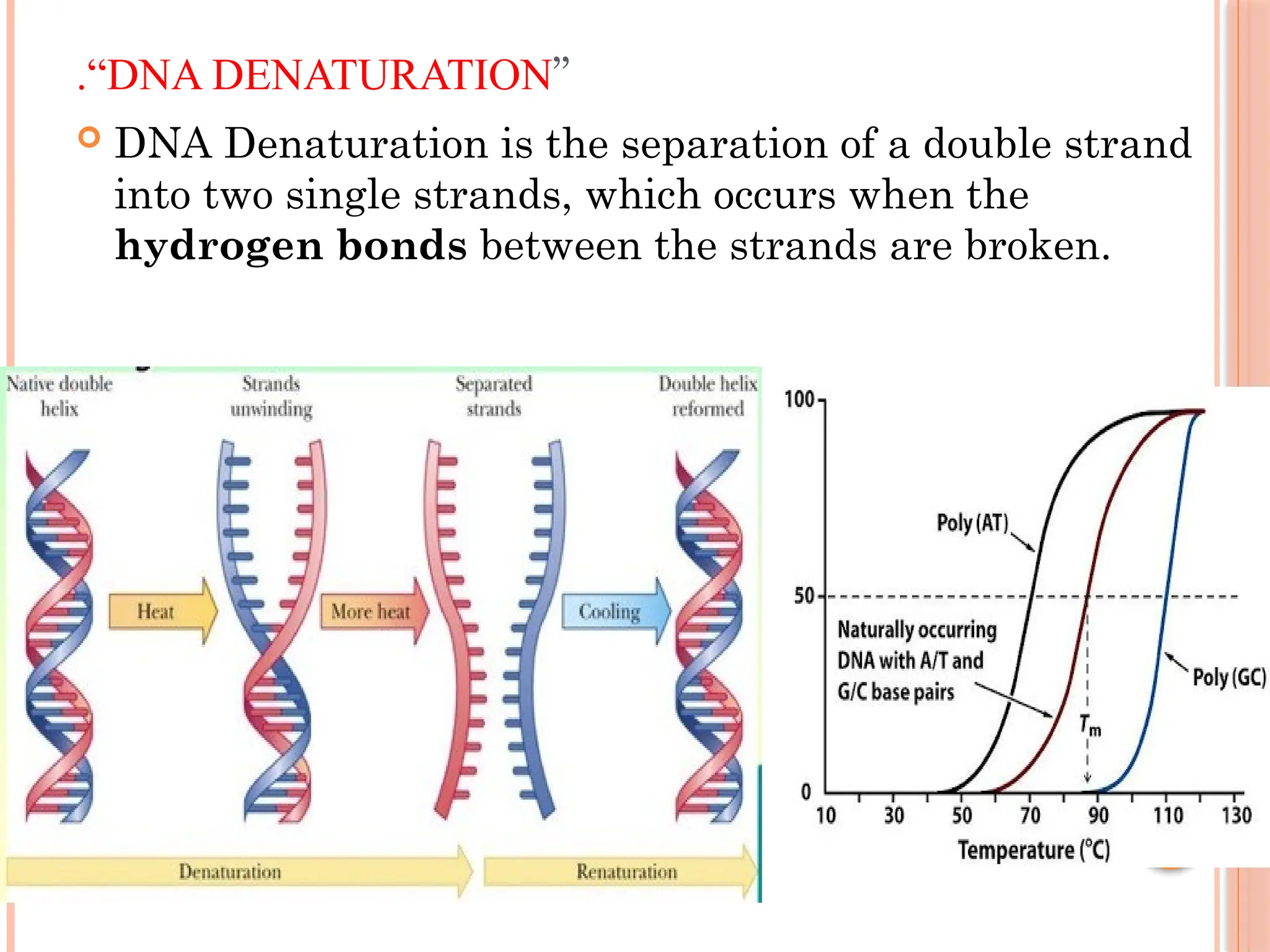
The document discusses the structure and function of DNA, emphasizing its role in encoding genetic information and its unique double-helix formation described by Watson and Crick in 1953. DNA consists of nucleotides and features a phosphate-sugar backbone, with base pairs that include adenine, thymine, guanine, and cytosine. Additionally, it highlights DNA's organization in eukaryotic, prokaryotic cells, and viruses, as well as the concept of denaturation where the DNA strands separate.