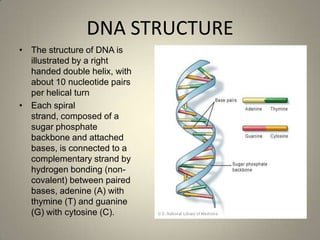
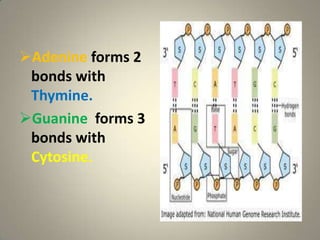
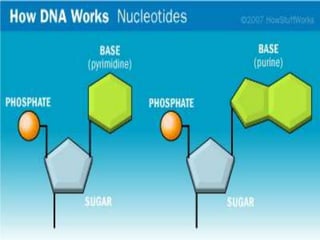
DNA stores genetic information that controls protein production and organism biochemistry. It has a double helix structure, with strands composed of sugar-phosphate backbones and attached nucleotide bases that pair through hydrogen bonding between adenine and thymine and between guanine and cytosine. Most DNA is located in the cell nucleus, where it is packaged into chromosomes, but mitochondria also contain a small amount of mitochondrial DNA.