1. The document describes how to use flame atomic absorption spectroscopy to determine the concentration of calcium in bottled water samples.
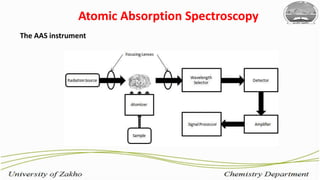
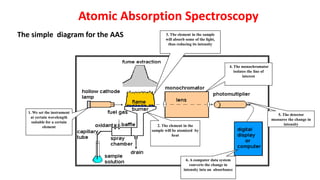
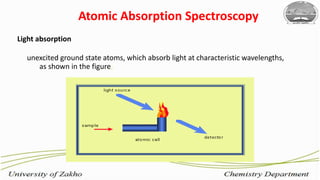
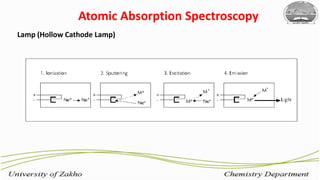
2. Flame atomic absorption spectroscopy works by aspirating and atomizing liquid samples using a flame, then measuring the absorption of light at characteristic wavelengths to detect specific metals.
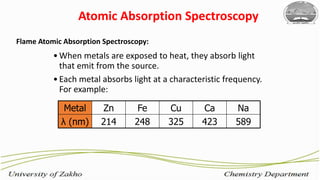
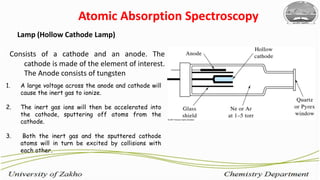
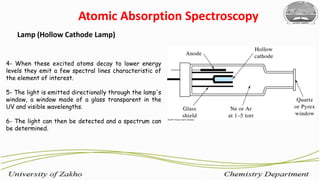
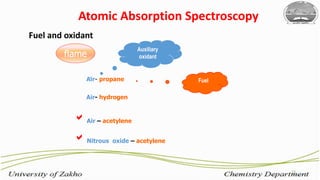
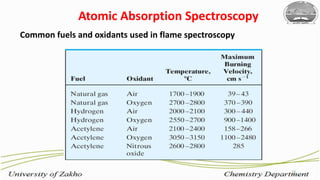
3. The technique requires samples to be aspirated and mixed with combustible gases and ignited in a flame between 2100-2800°C to atomize the metals, which will absorb light from a hollow cathode lamp at wavelengths specific to each metal.