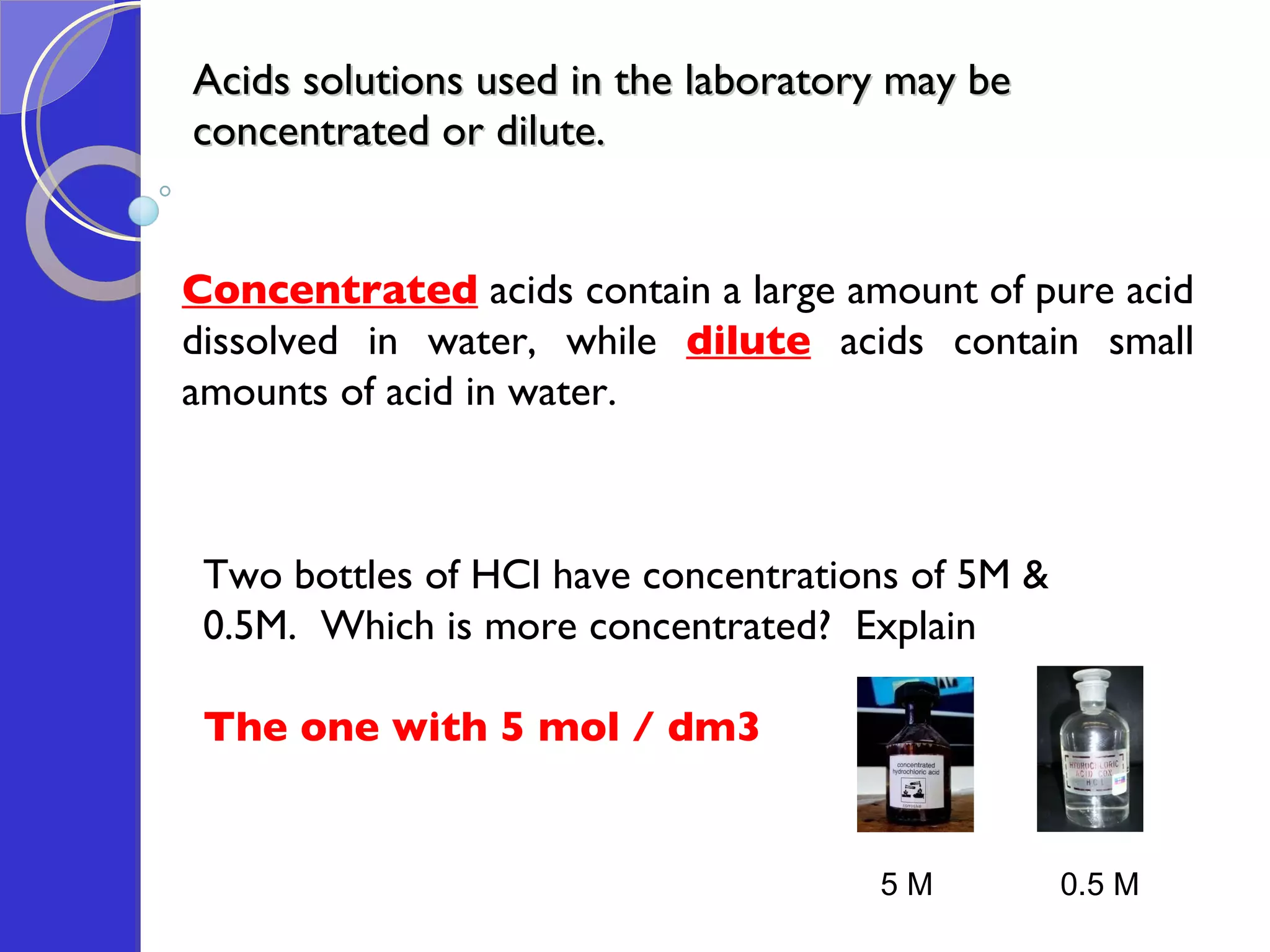
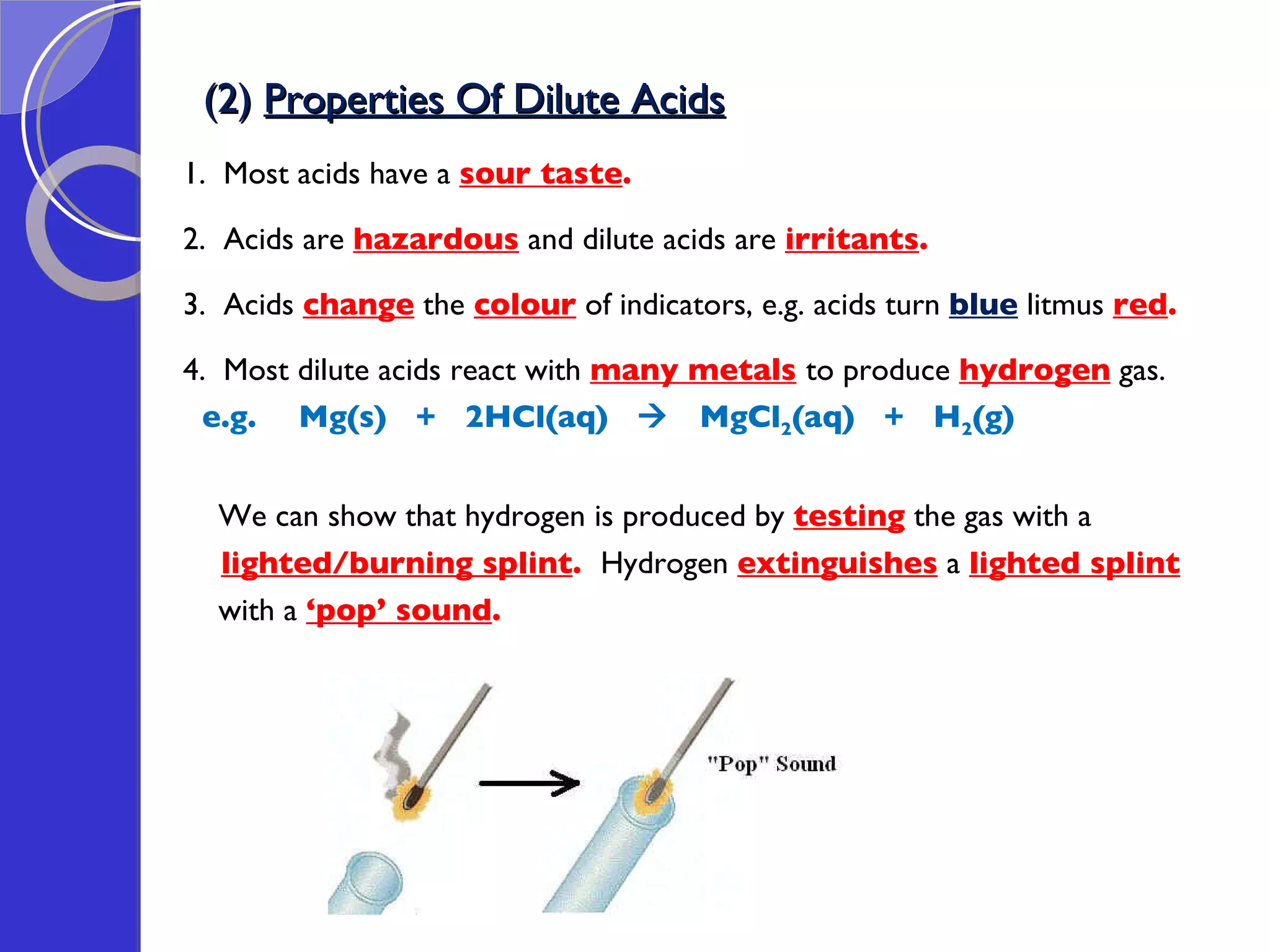
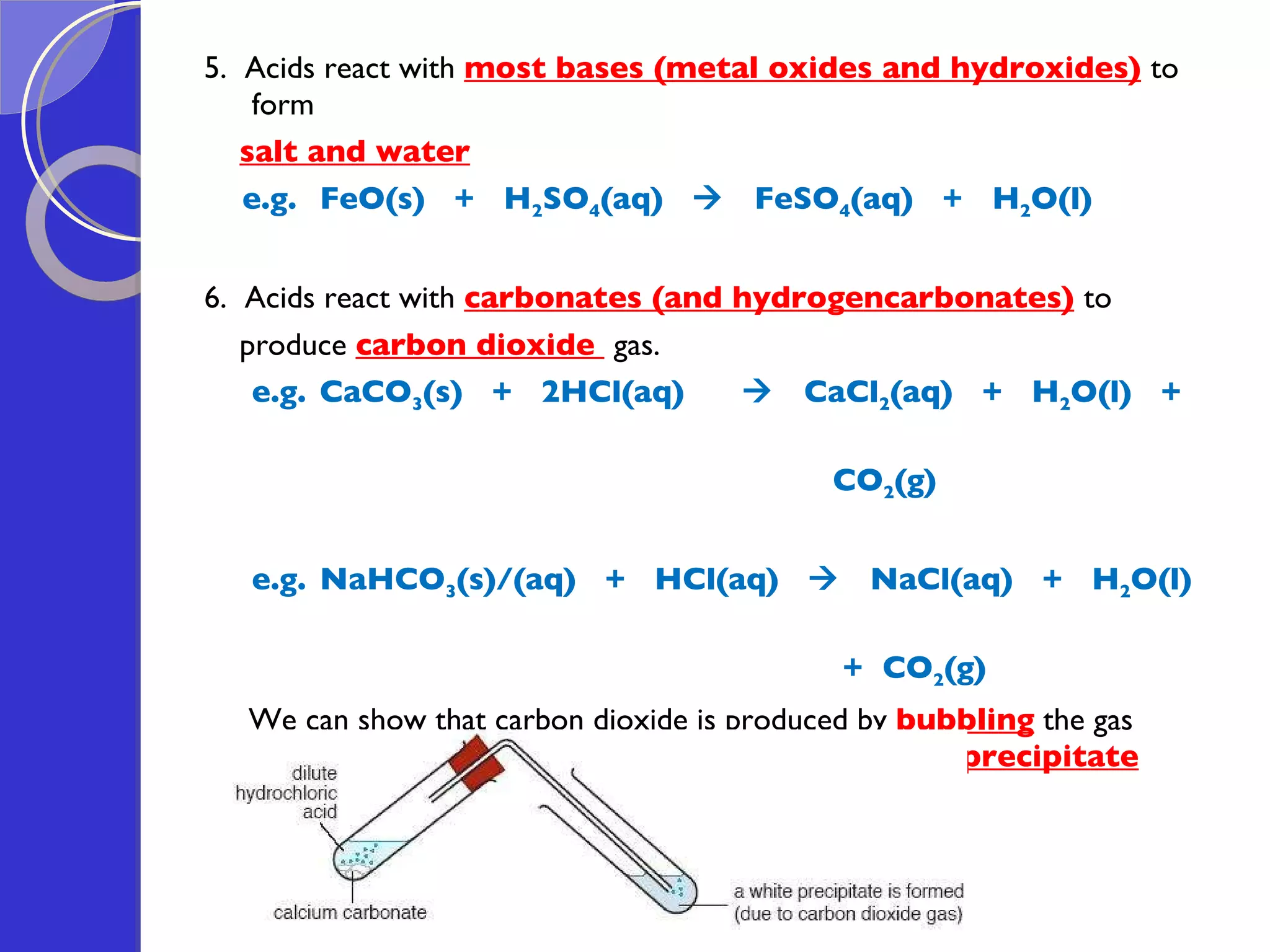
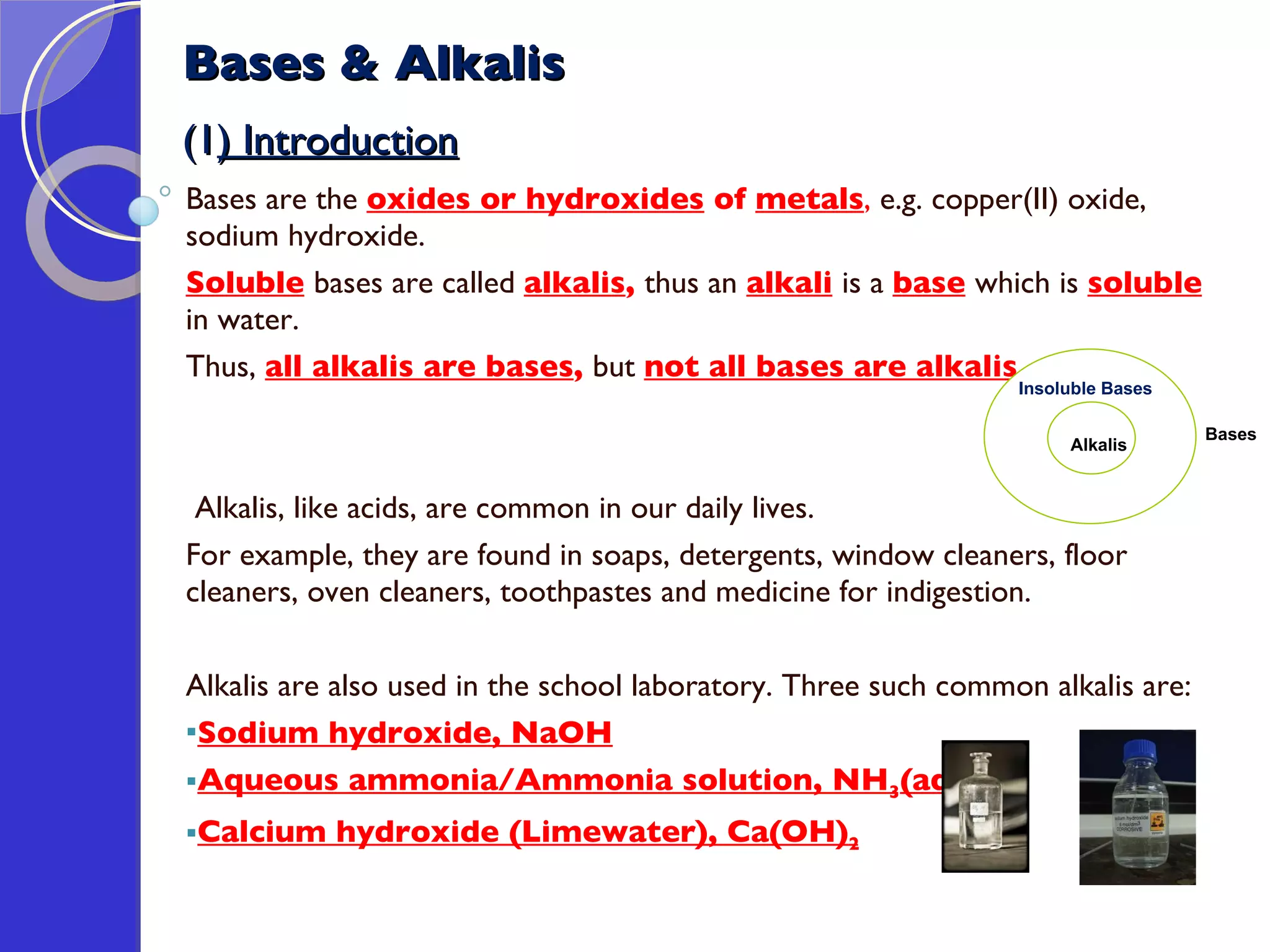
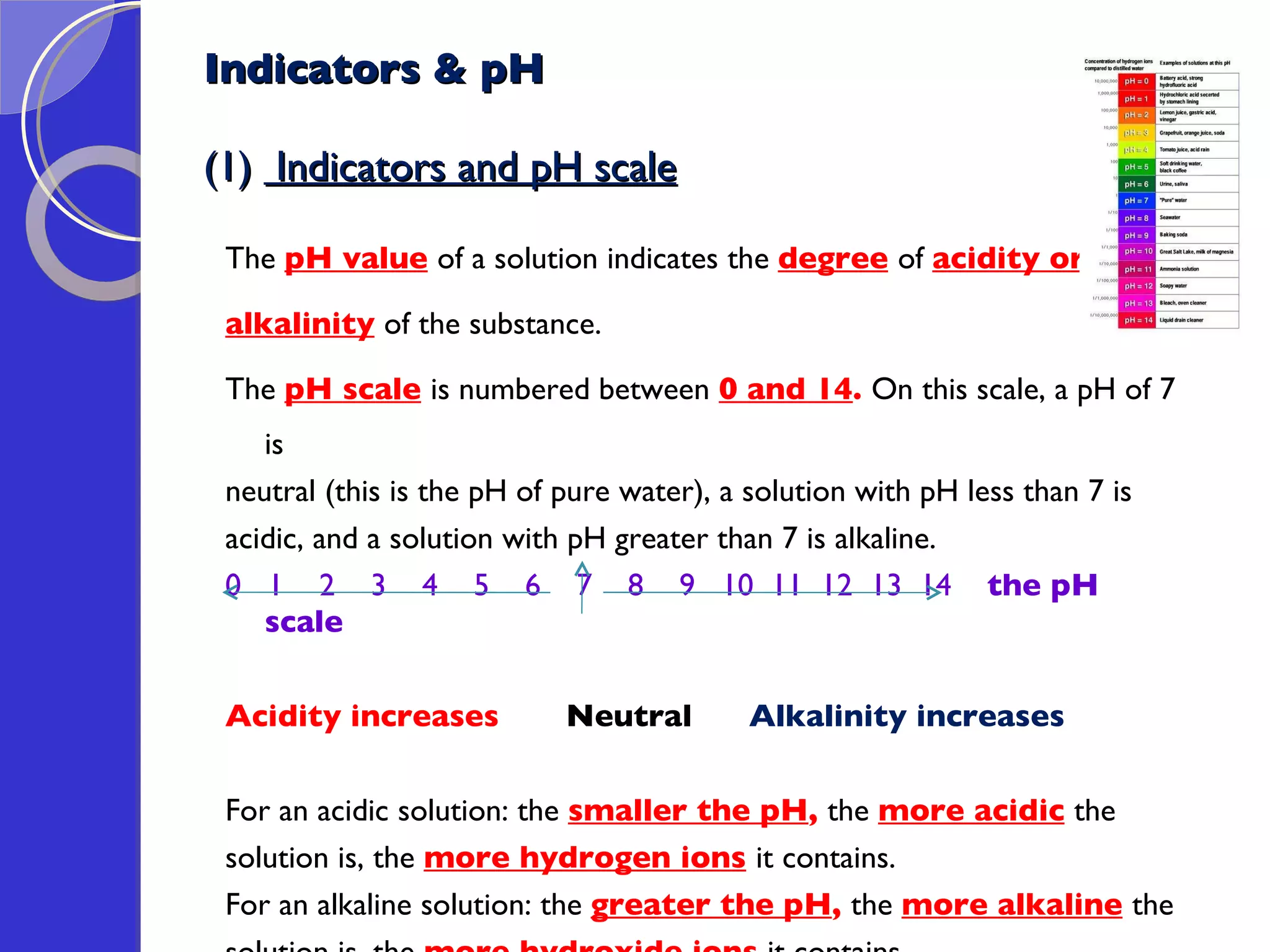
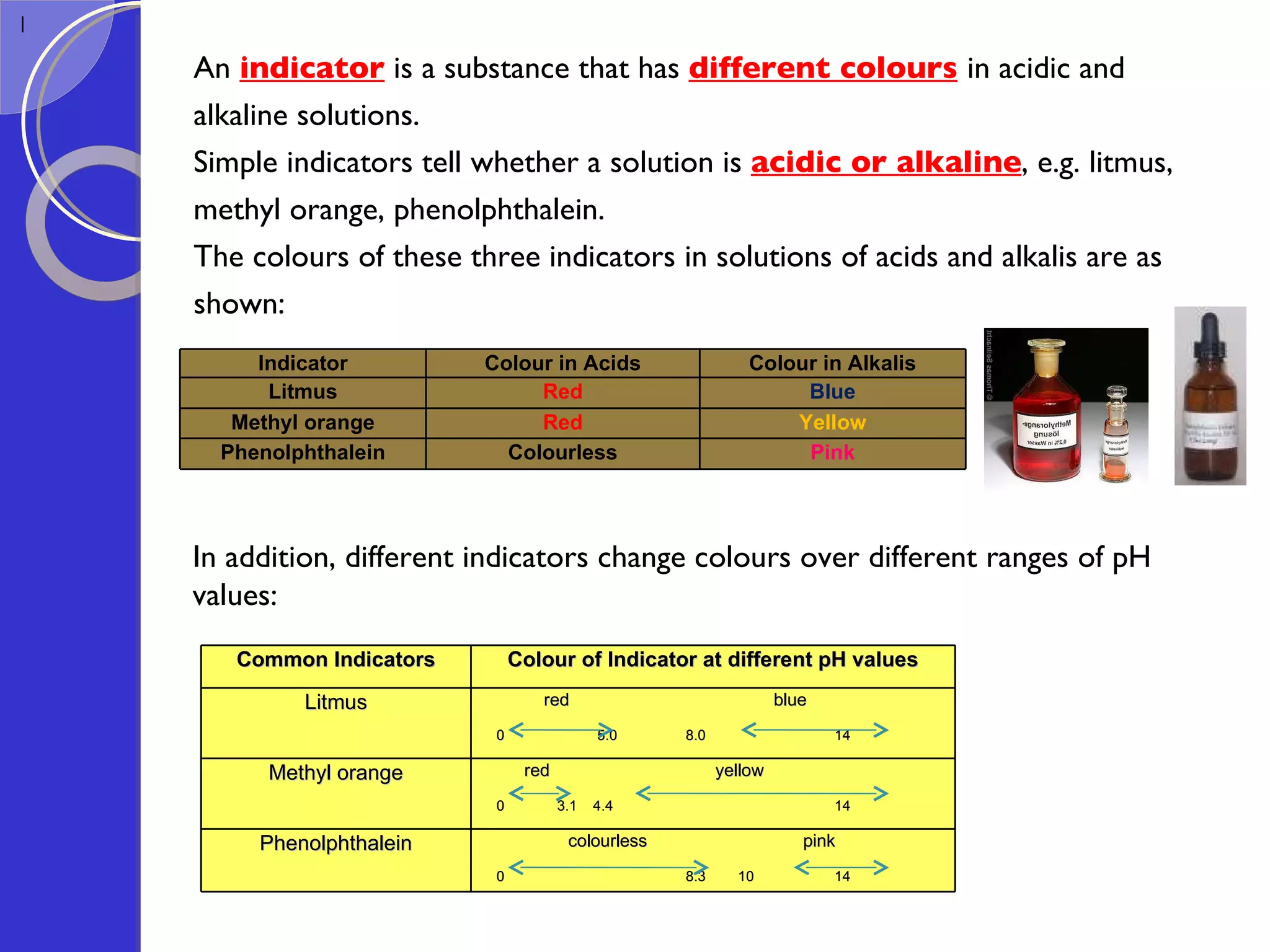
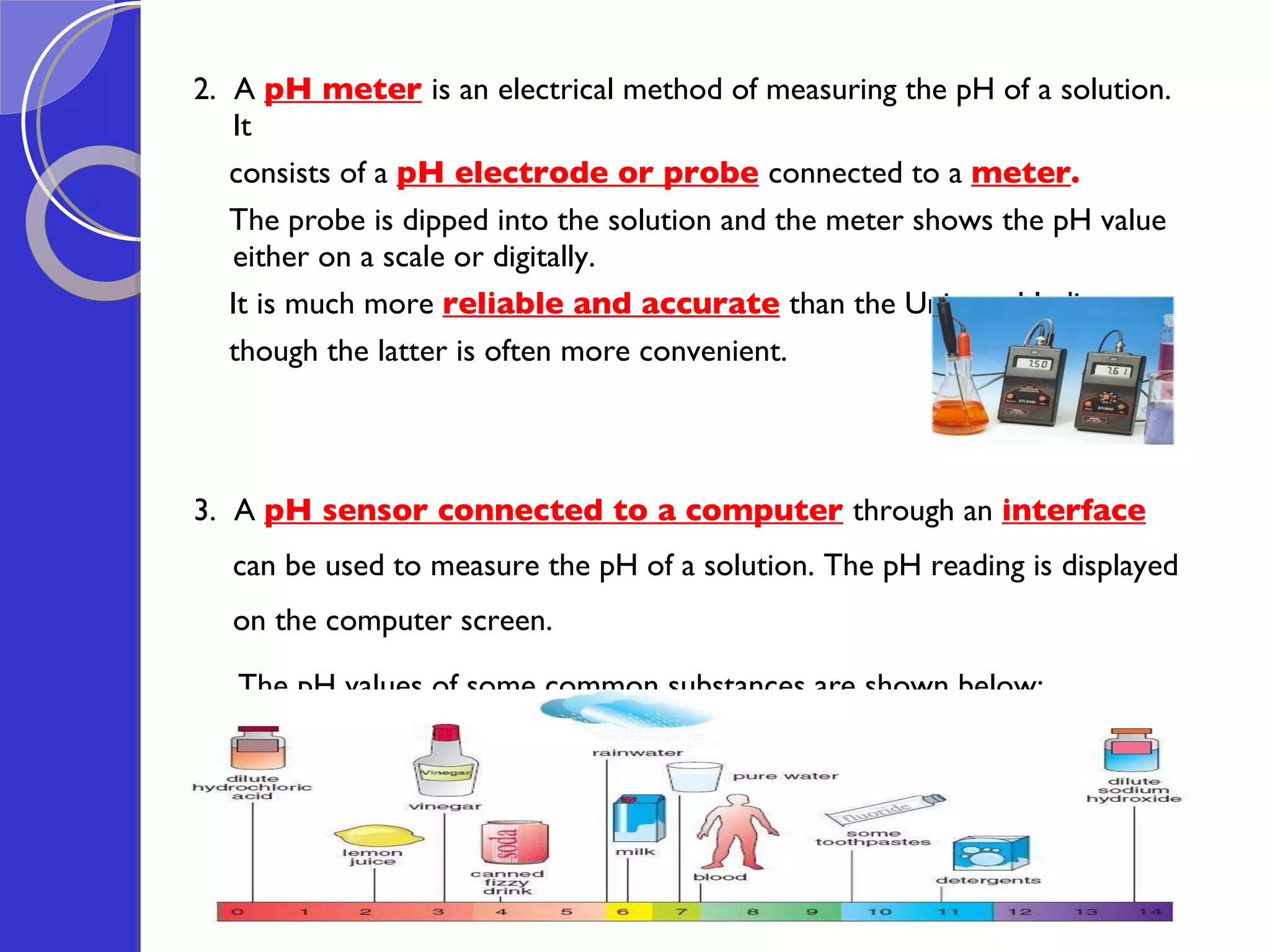
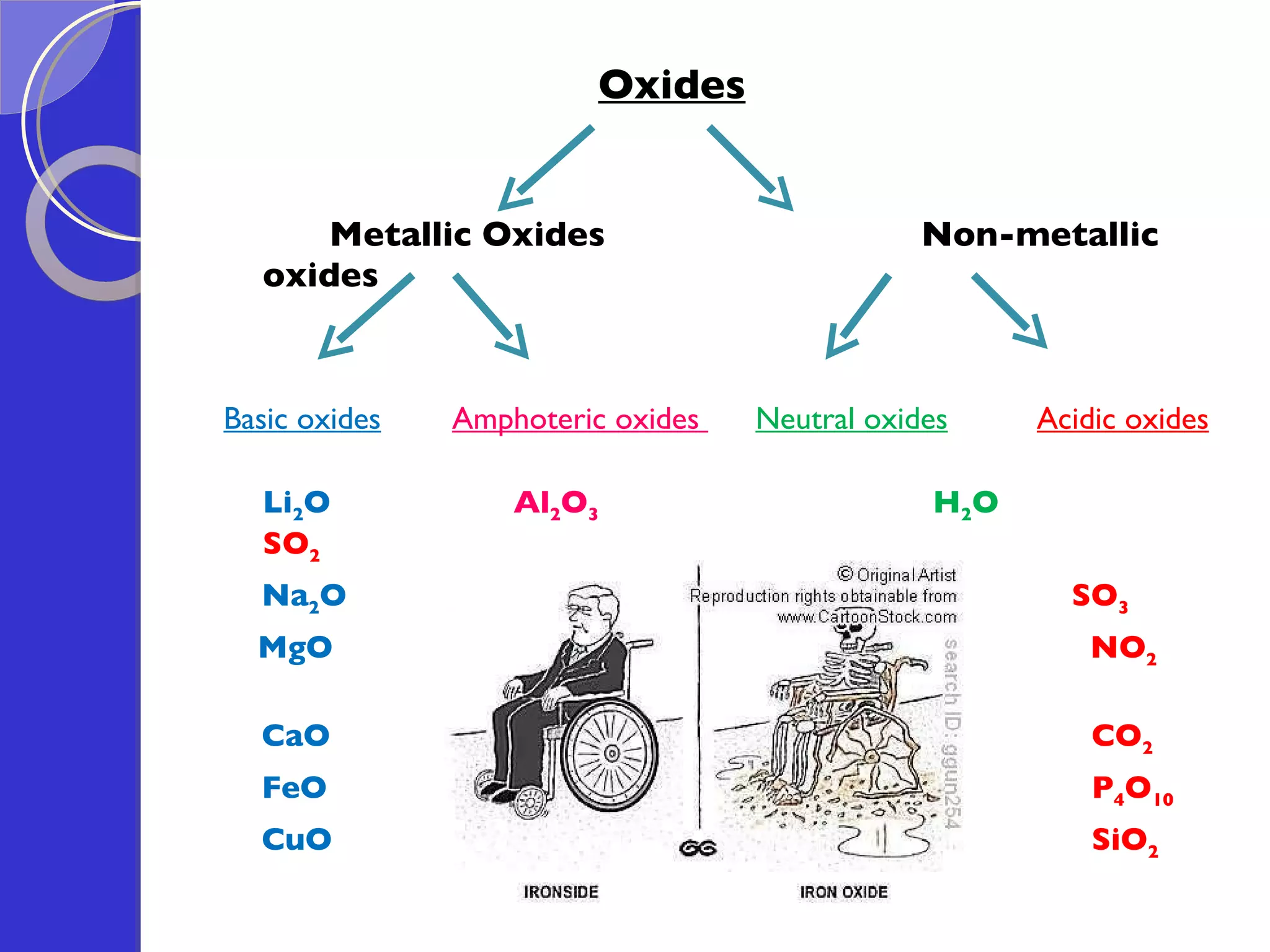
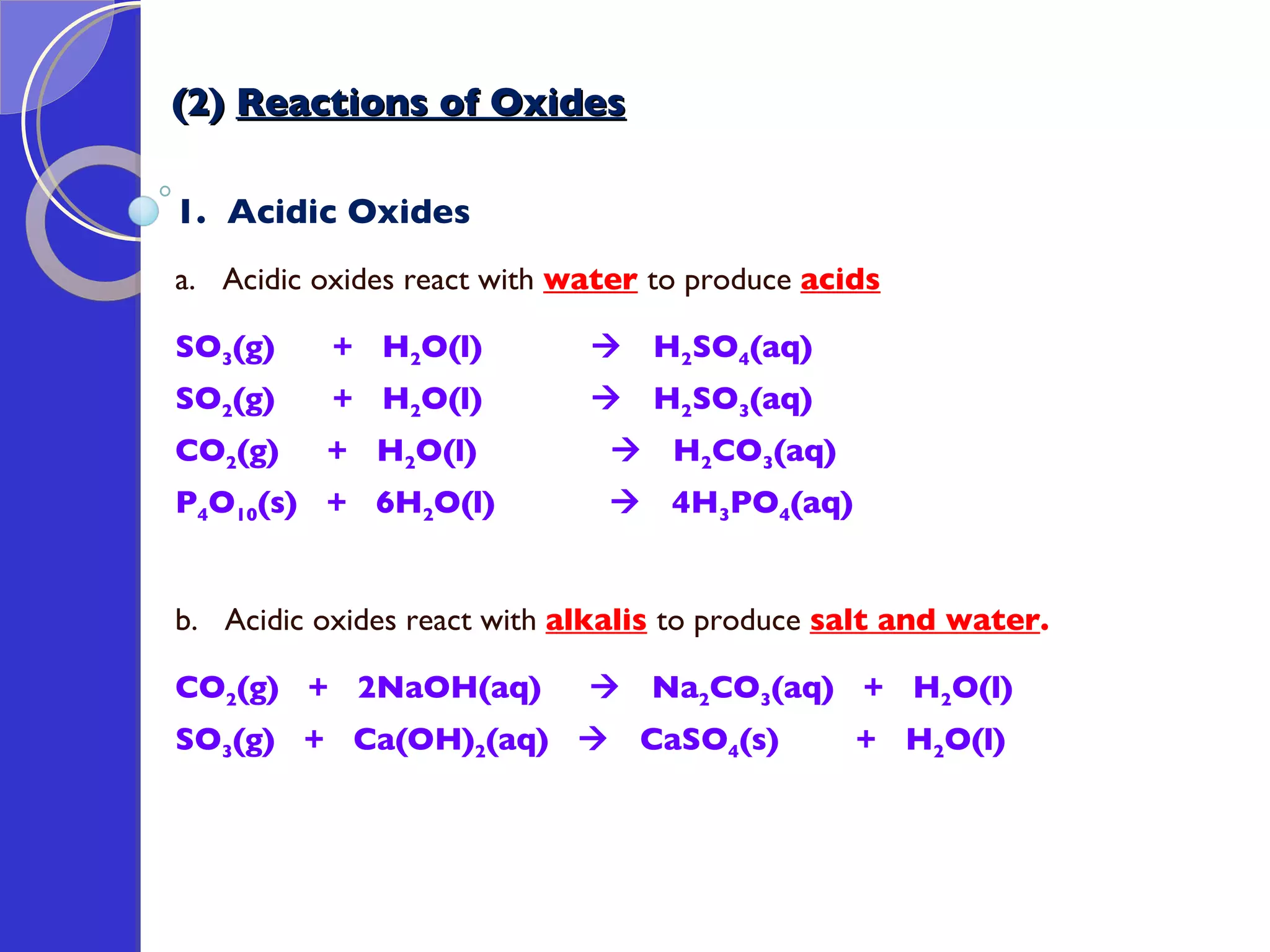
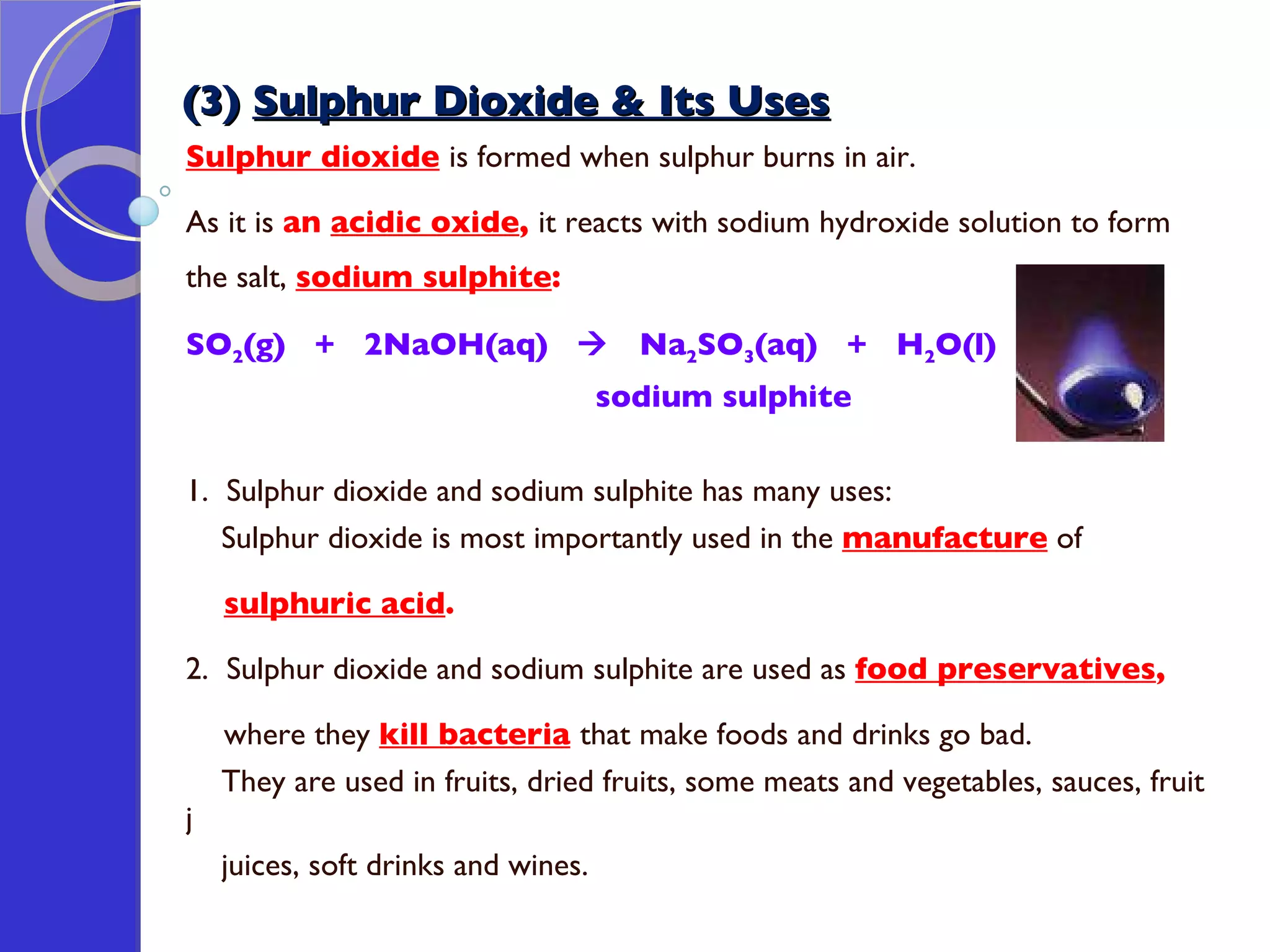
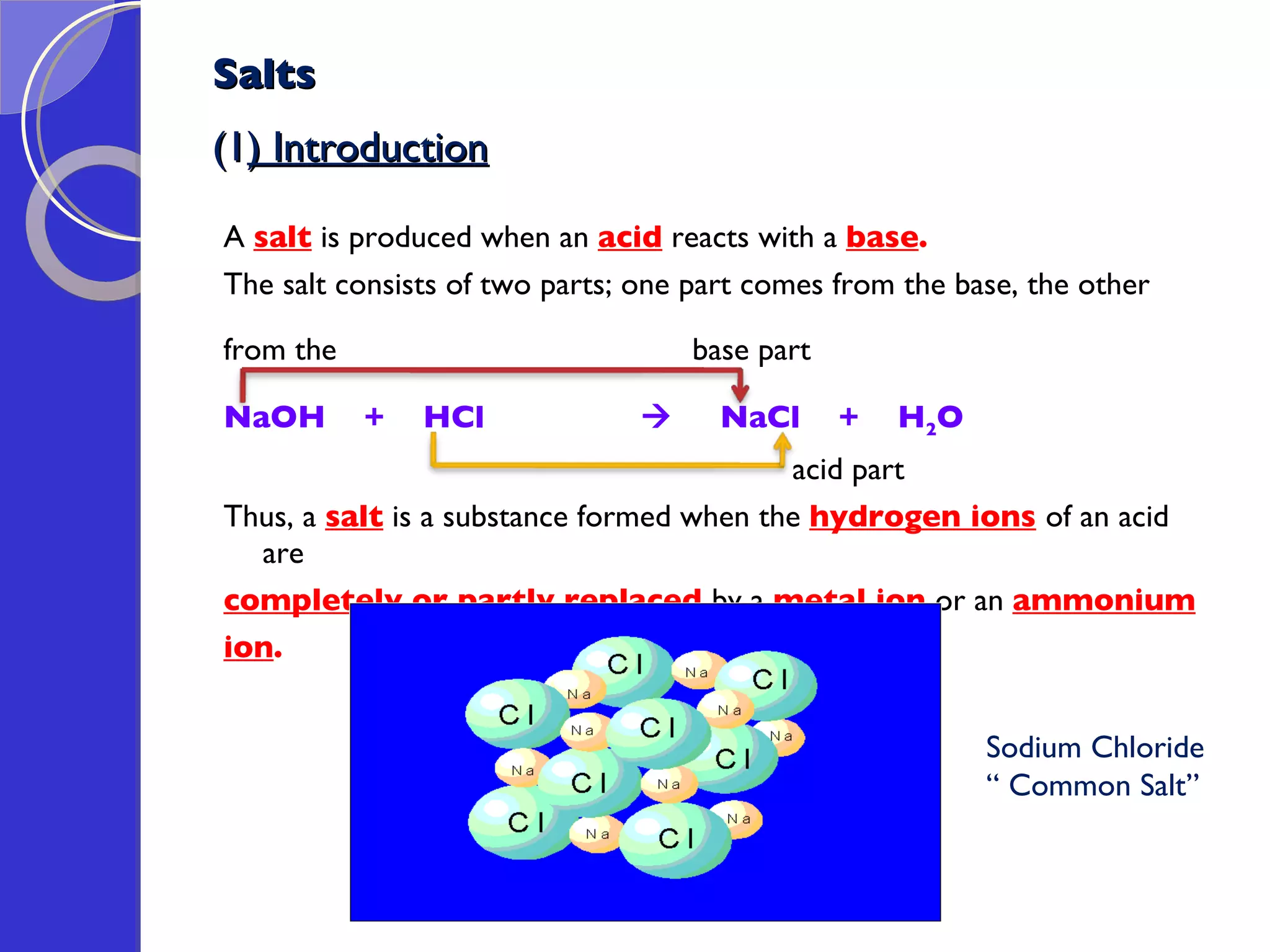
This document provides information about acids and bases, including their properties and reactions. It defines acids as substances that produce hydrogen ions in aqueous solution, and bases as metal oxides or hydroxides. Strong acids are fully ionized in water, while weak acids are only partially ionized. The strength of an acid does not relate to its concentration. Common uses of acids include battery electrolytes, rust removal, and food preservation.






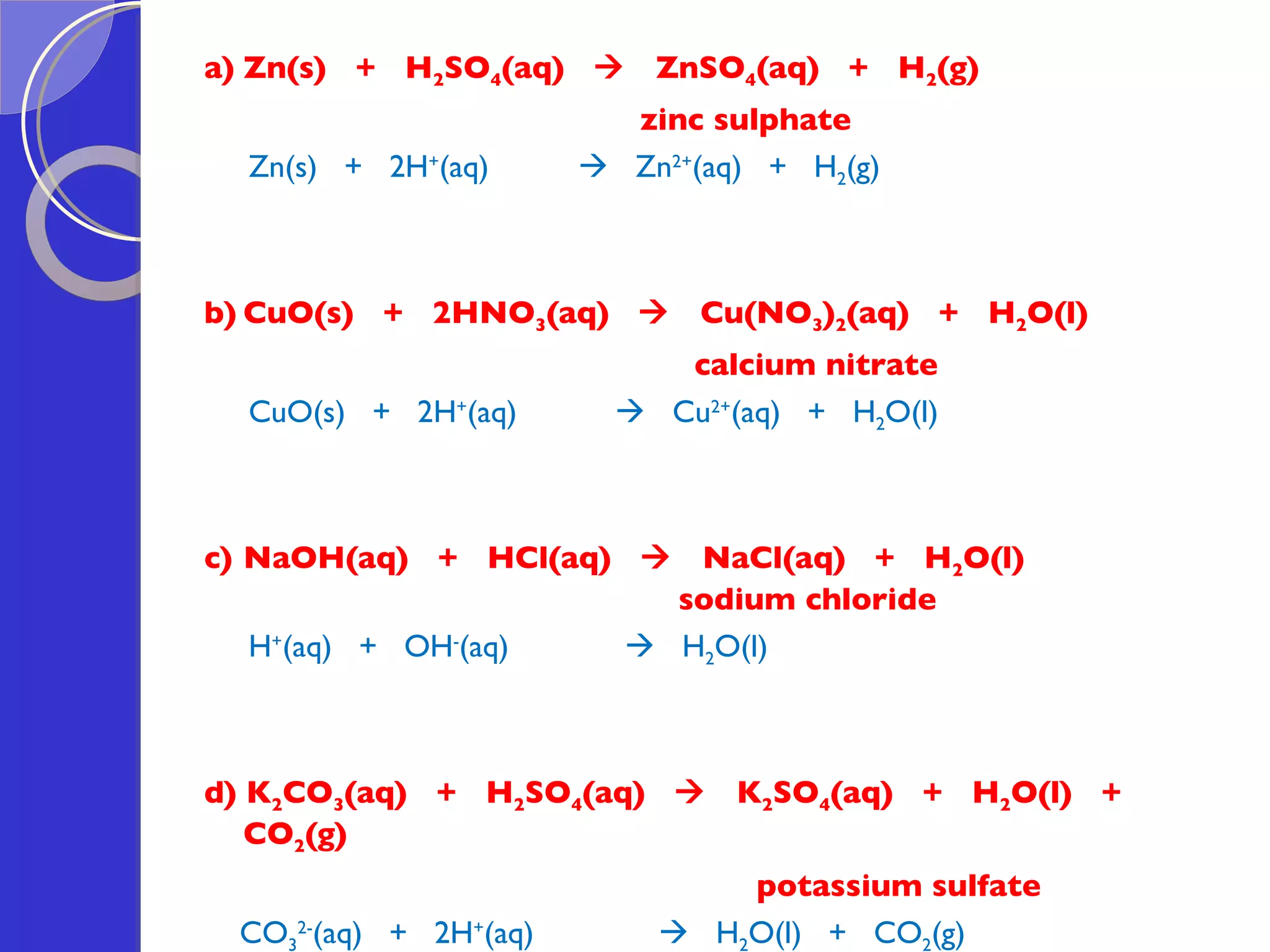




































![Exercise: A student attempted to prepare lead(II) sulphate in the laboratory by reacting lead(II) oxide and dilute sulphuric acid. Would he succeed in the preparation? Explain why. No, he would not succeed. This is because once the product, lead(II) sulphate is formed during the reaction, being an insoluble salt (insoluble in water), it would coat onto the unreacted lead(II) oxide, thus inhibiting further reaction between lead(II) oxide and dilute sulphuric acid. [in fact, since lead(II) sulphate is an insoluble salt, the precipitation method should be used => e.g. using aqueous lead(II) nitrate and aqueous sodium sulphate as reactants.]](https://image.slidesharecdn.com/acidsandbases-091015083202-phpapp02/75/Acids-And-Bases-67-2048.jpg)