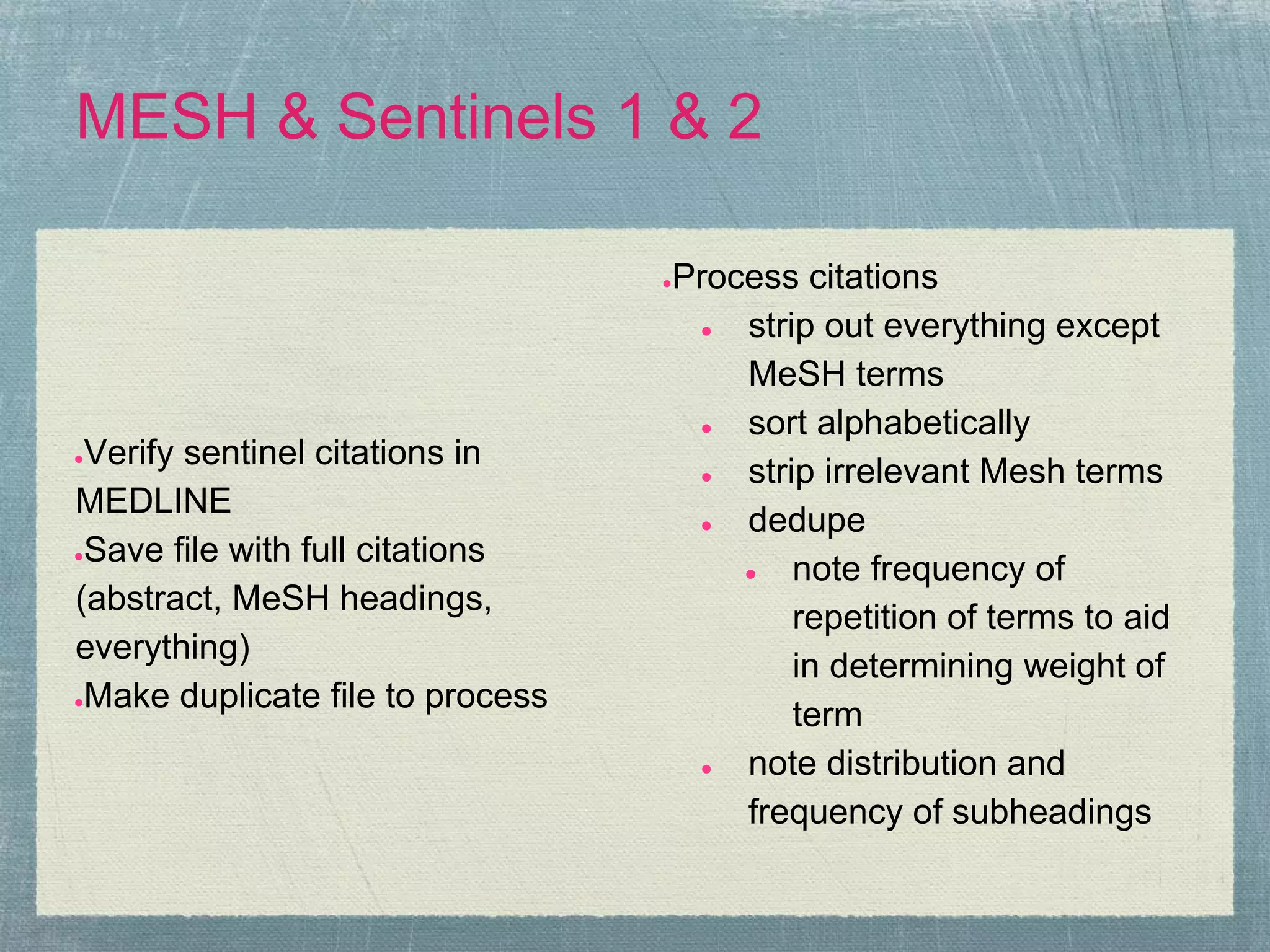
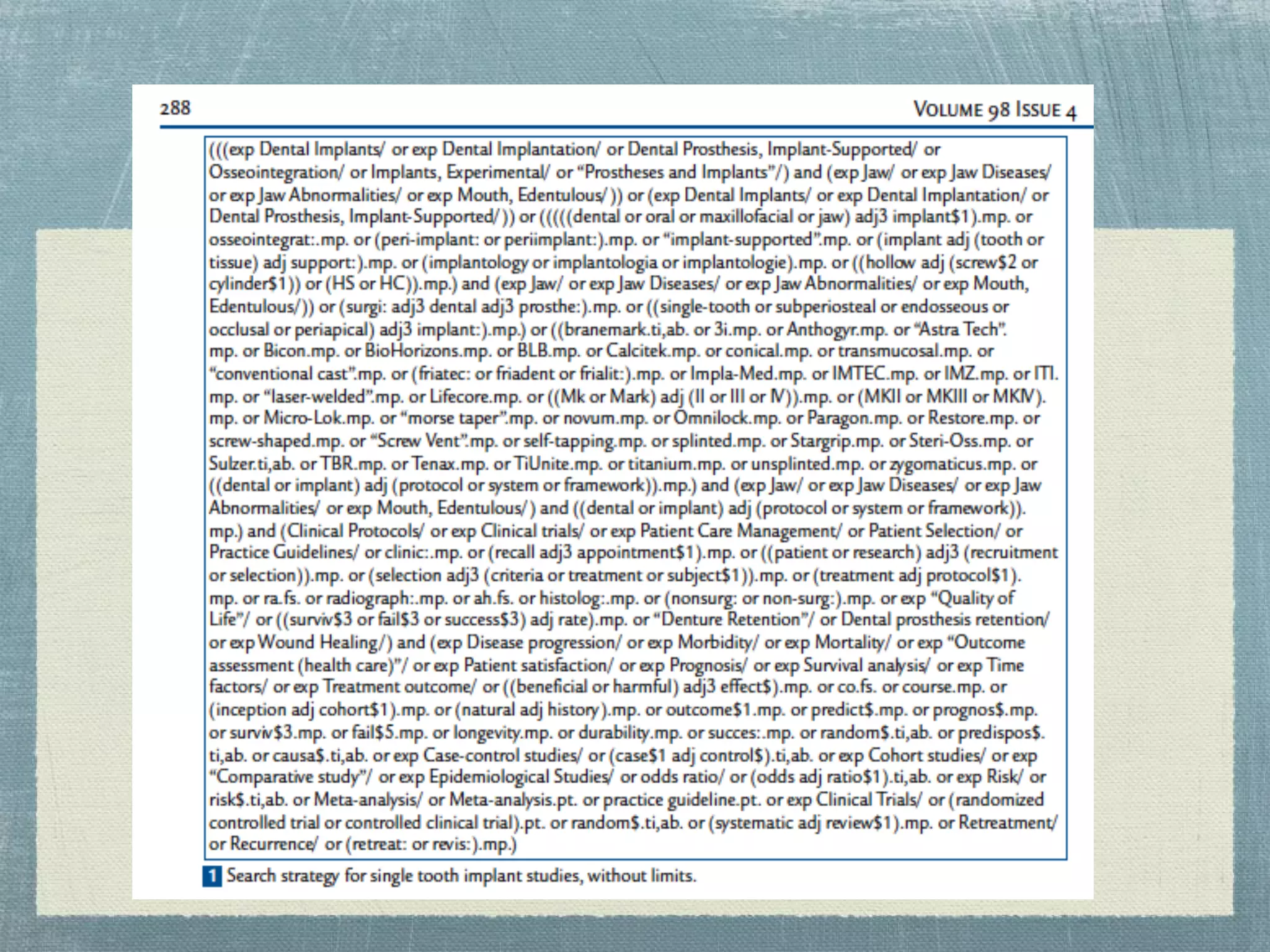
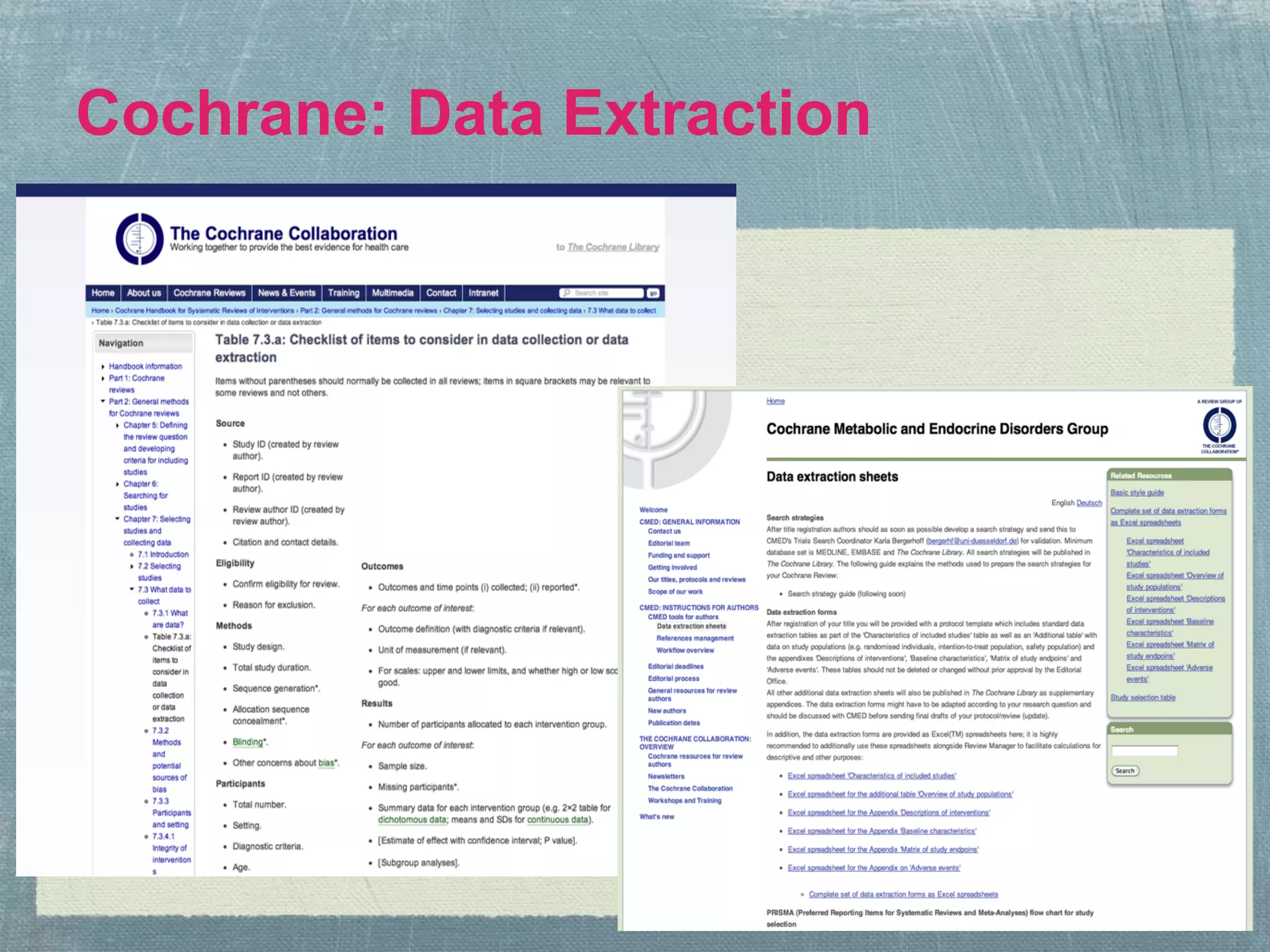
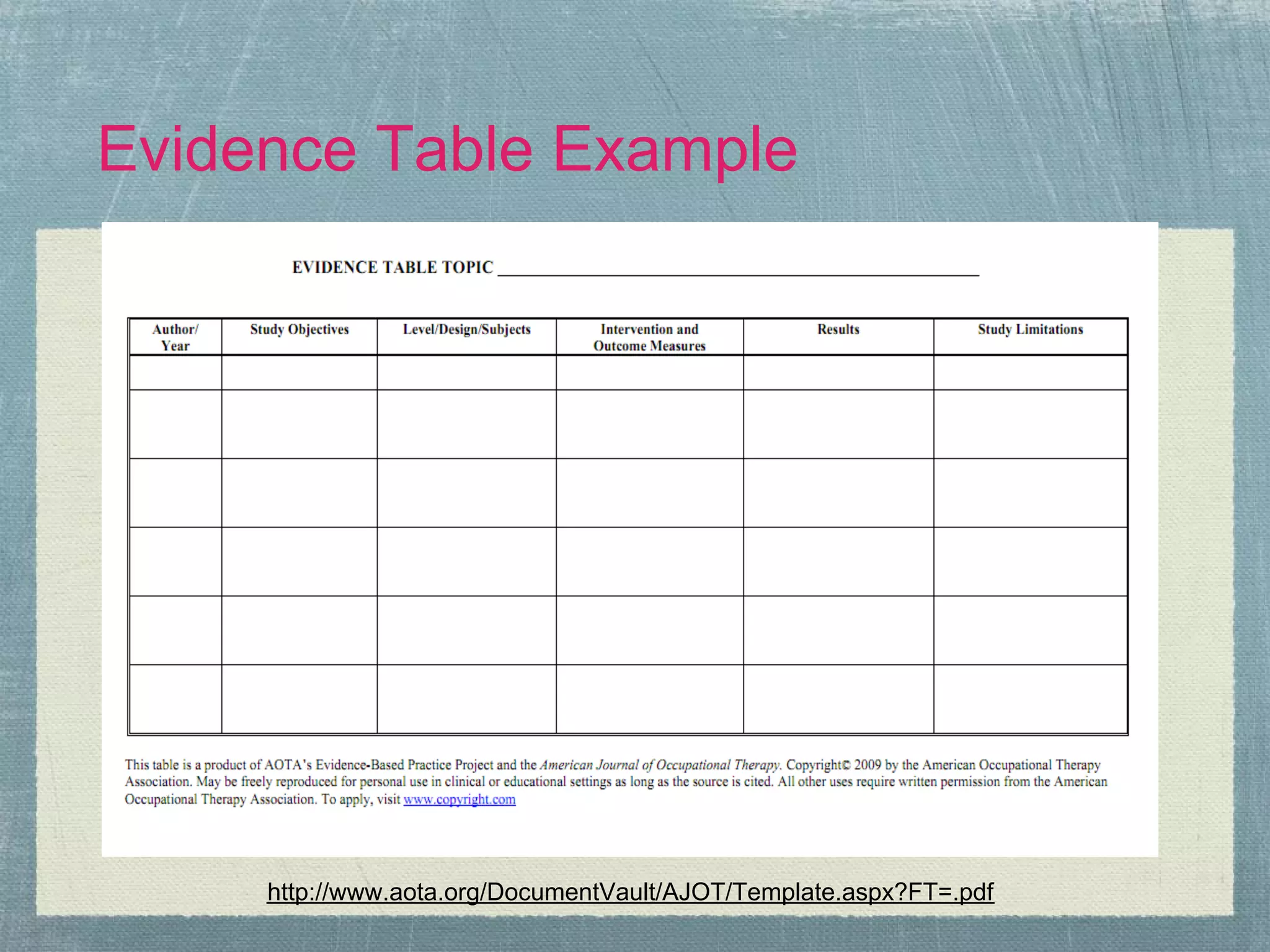
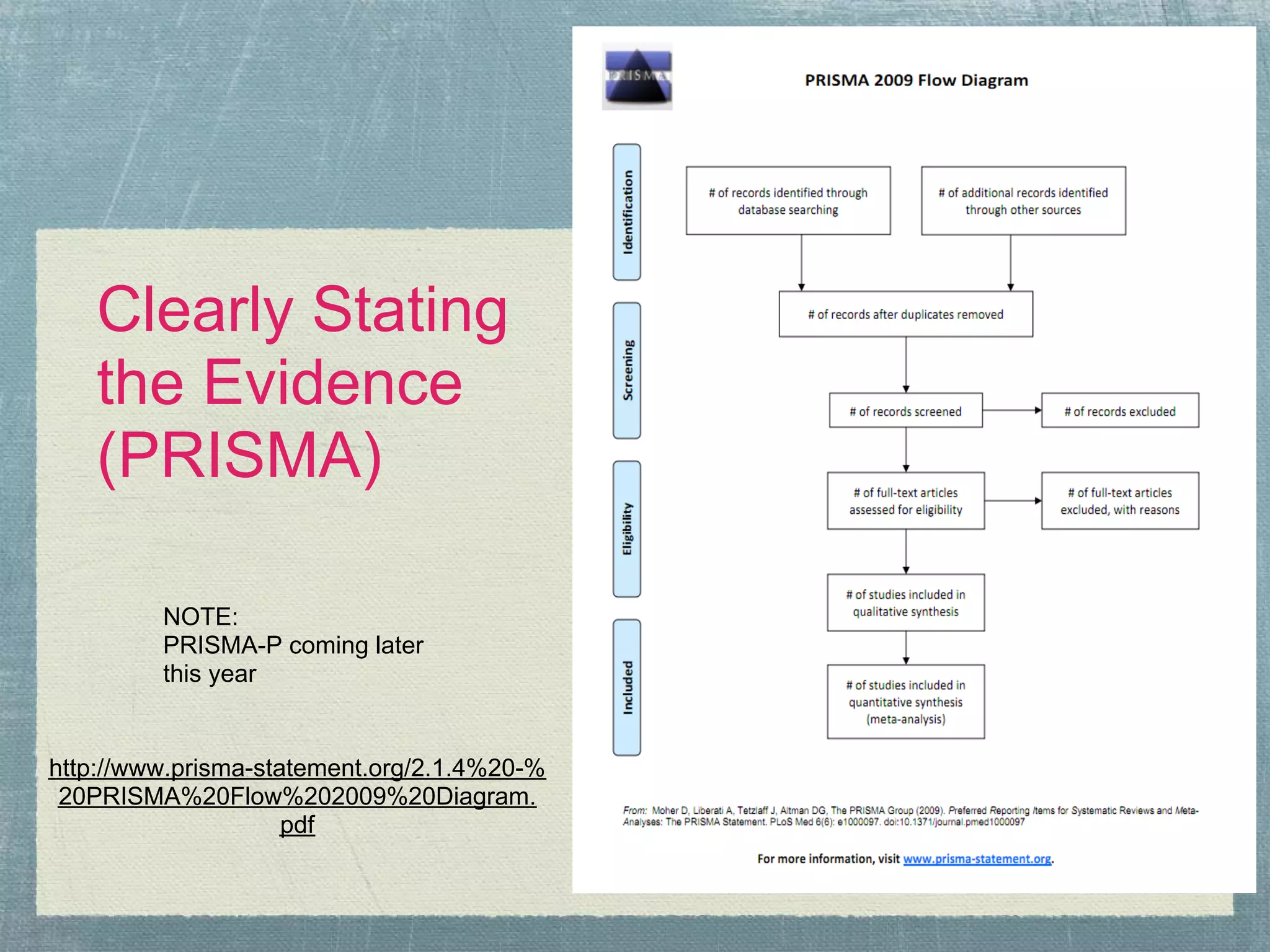
The document outlines the systematic review process, emphasizing its purpose, methodology, and the role of various team members, including librarians and clinical experts. It differentiates between evidence-based healthcare and systematic reviews, detailing the steps involved in conducting and reporting reviews. Additionally, it discusses best practices for search strategies, data extraction, and adherence to publication standards.




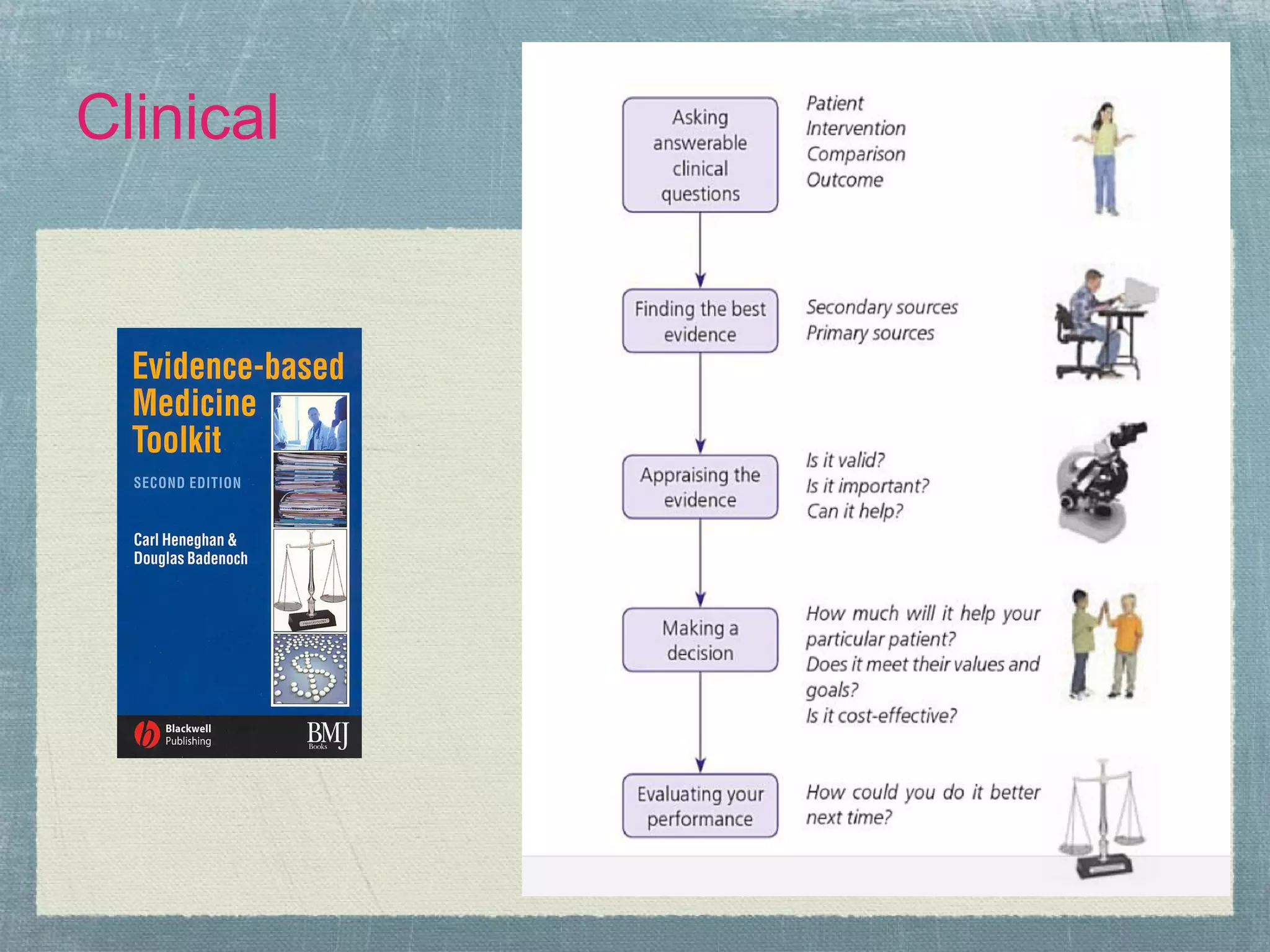



![What is Evidence-Based Healthcare?
●Short version:
●‘Make your [clinical] decisions based on the
best evidence available, integrated with your
clinical judgment. That’s all it means. The best
evidence, whatever that is.’
● Paraphrased from Dr. Ismail in
conversation, circa 2003.](https://image.slidesharecdn.com/systematicreviewteamsprocessesexperiences-120420142505-phpapp02/75/Systematic-review-teams-processes-experiences-10-2048.jpg)
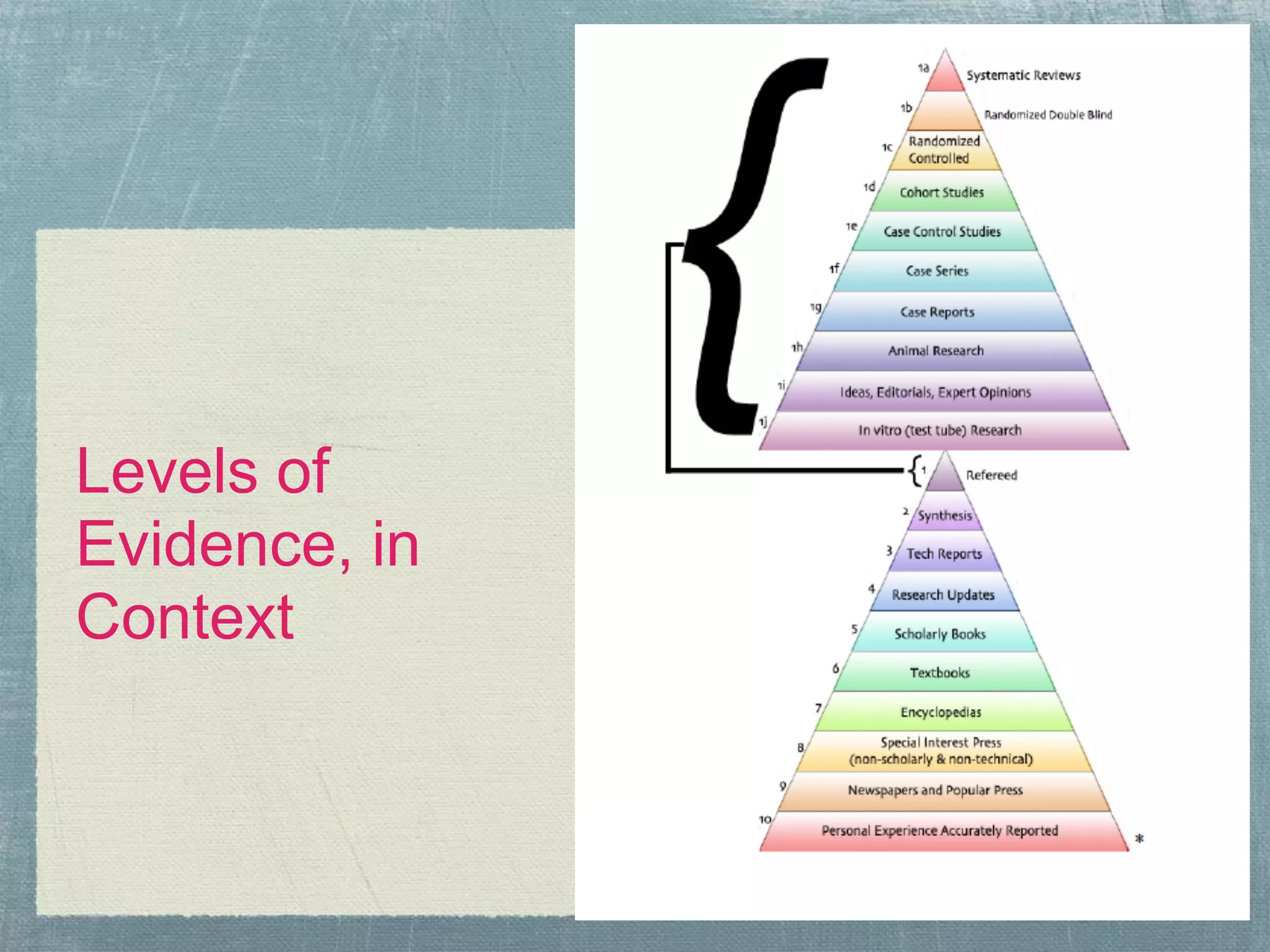

![Process & Methodology, Overview
Team meets
●
● Define topic, overview literature base, suggest inclusion/exclusion
criteria, discuss methodology & timeline.
Librarian
●
● Generates data for the team
● FRIAR/MEMORABLE/SECT
● Topic experts collaborate
Topic experts
●
● Review data at 3-4 levels (title, abstract, article, [request additional
information]), achieve consensus
● Handsearching (librarian generates list, experts implement)
● Determine level of evidence for remaining research
● Generate review tables
Share findings (Publication)
●
● Strength of evidence available (strong, weak, inadequate); suggest
directions for future research to fill gaps in research base](https://image.slidesharecdn.com/systematicreviewteamsprocessesexperiences-120420142505-phpapp02/75/Systematic-review-teams-processes-experiences-13-2048.jpg)











![MESH Tip
●Earlier term mappings prior to
assignment of a MeSH term are often:
● presenting symptom or diagnosis
● anatomical area
●TMJD Example:
● TMJD = temporomandibular joint
disorder
● = (Temporomandibular joint
[anatomical area] + ("myofacial
pain" OR "Bone Diseases")
[presenting symptom OR
diagnosis]
Image: Frank Gaillard. Normal anatomy of the Temporomandibular joint. 14 Jan
2009. http://commons.wikimedia.org/wiki/File:Temporomandibular_joint.png](https://image.slidesharecdn.com/systematicreviewteamsprocessesexperiences-120420142505-phpapp02/75/Systematic-review-teams-processes-experiences-25-2048.jpg)