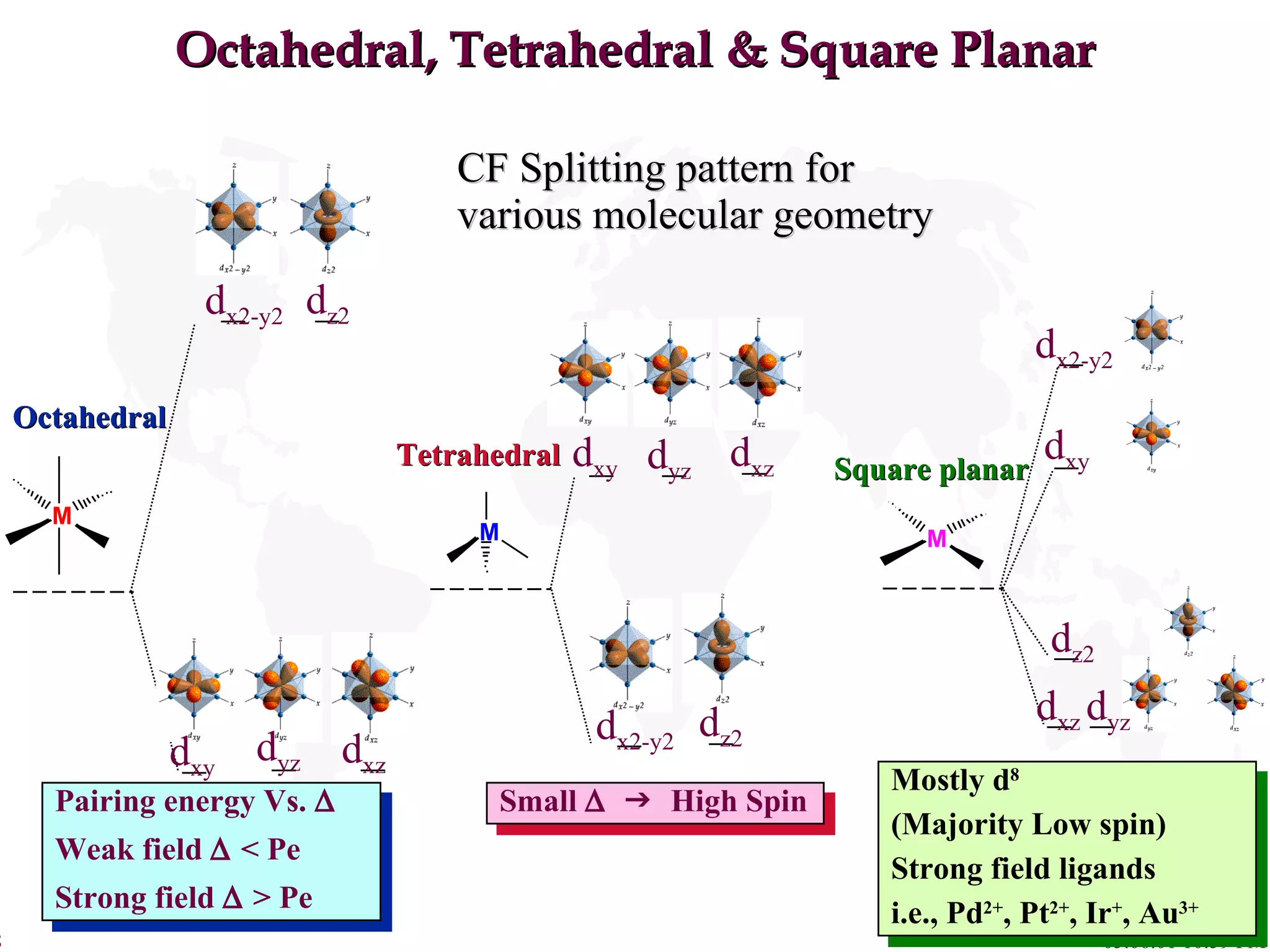
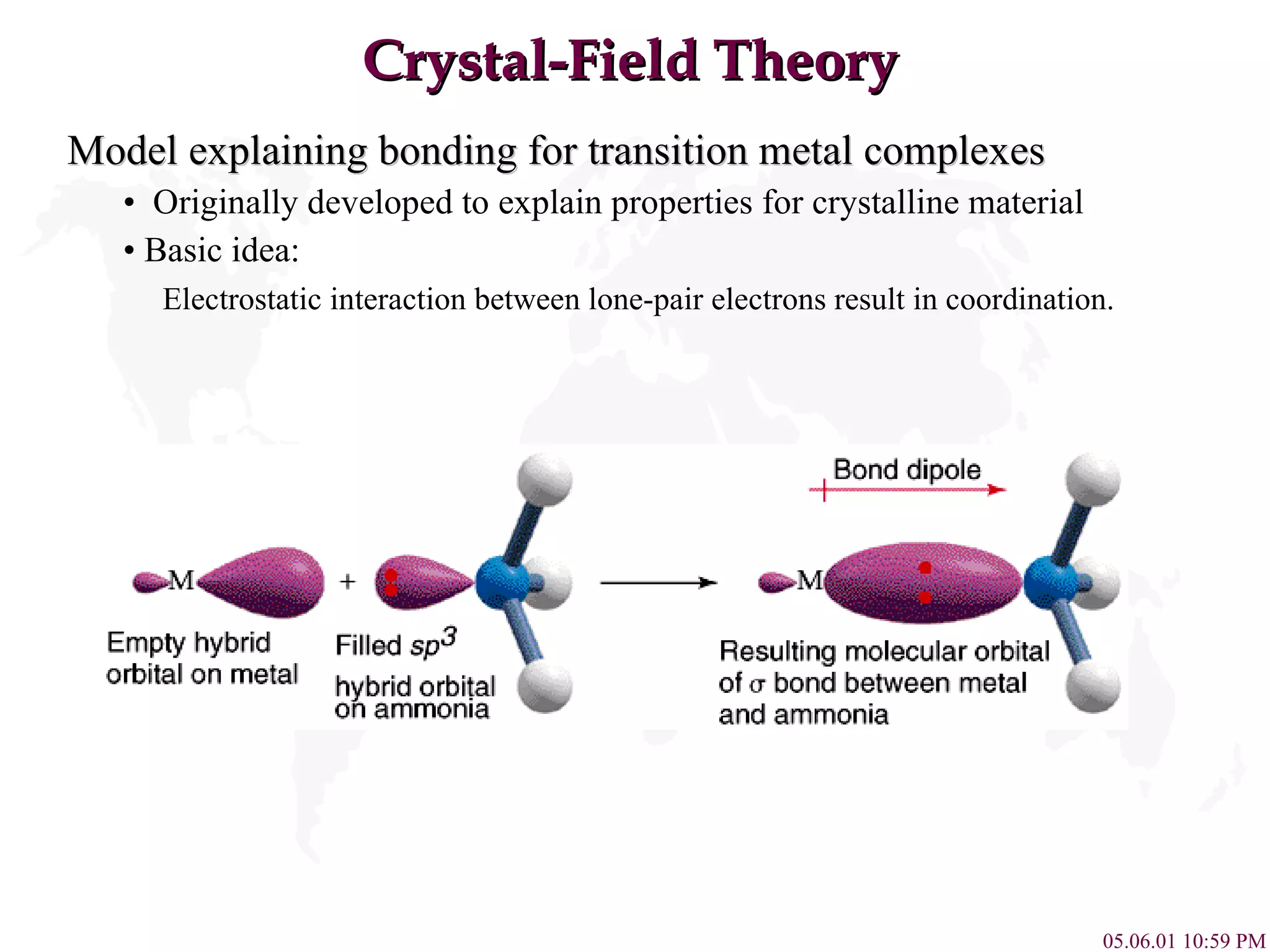
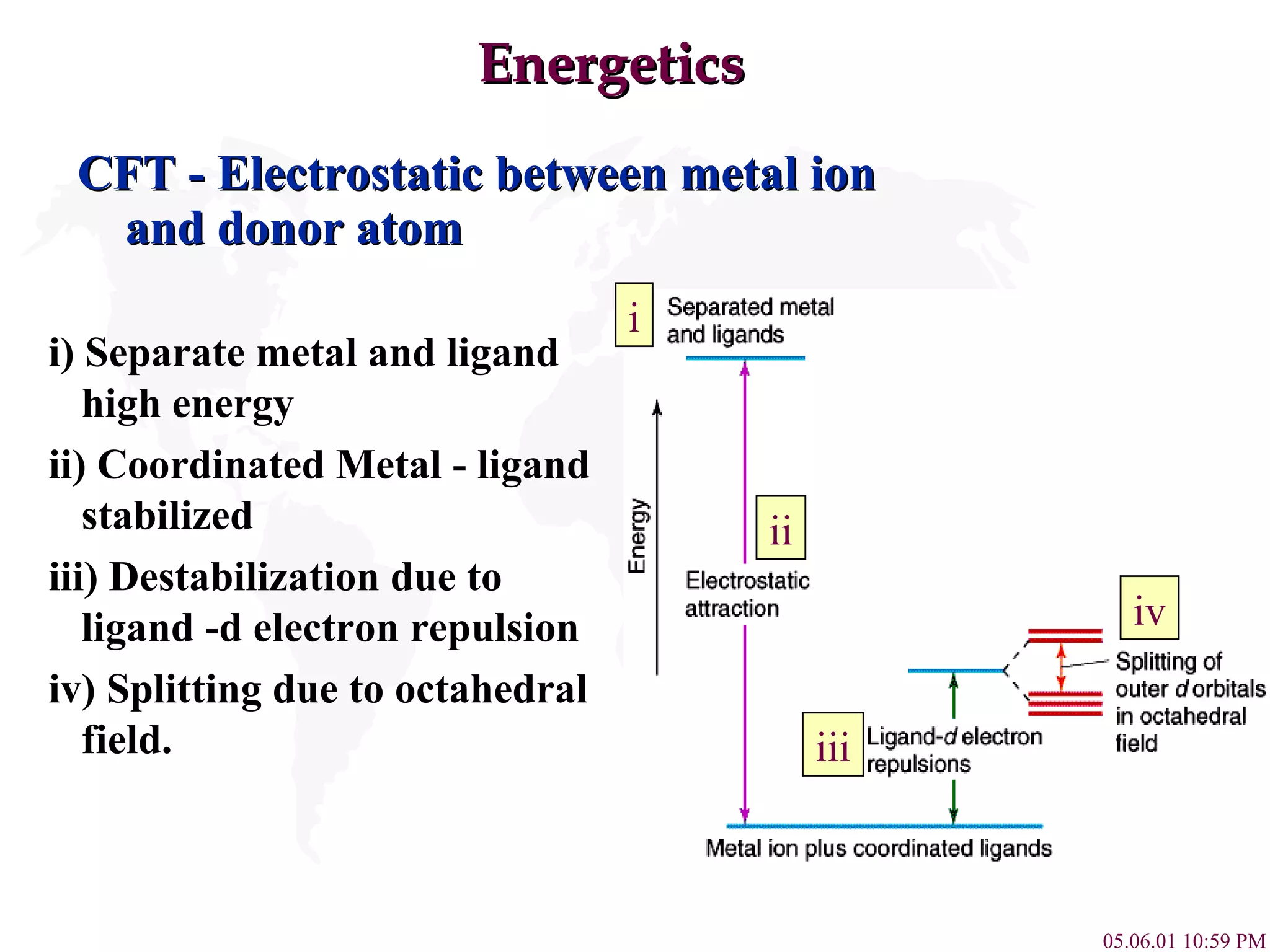
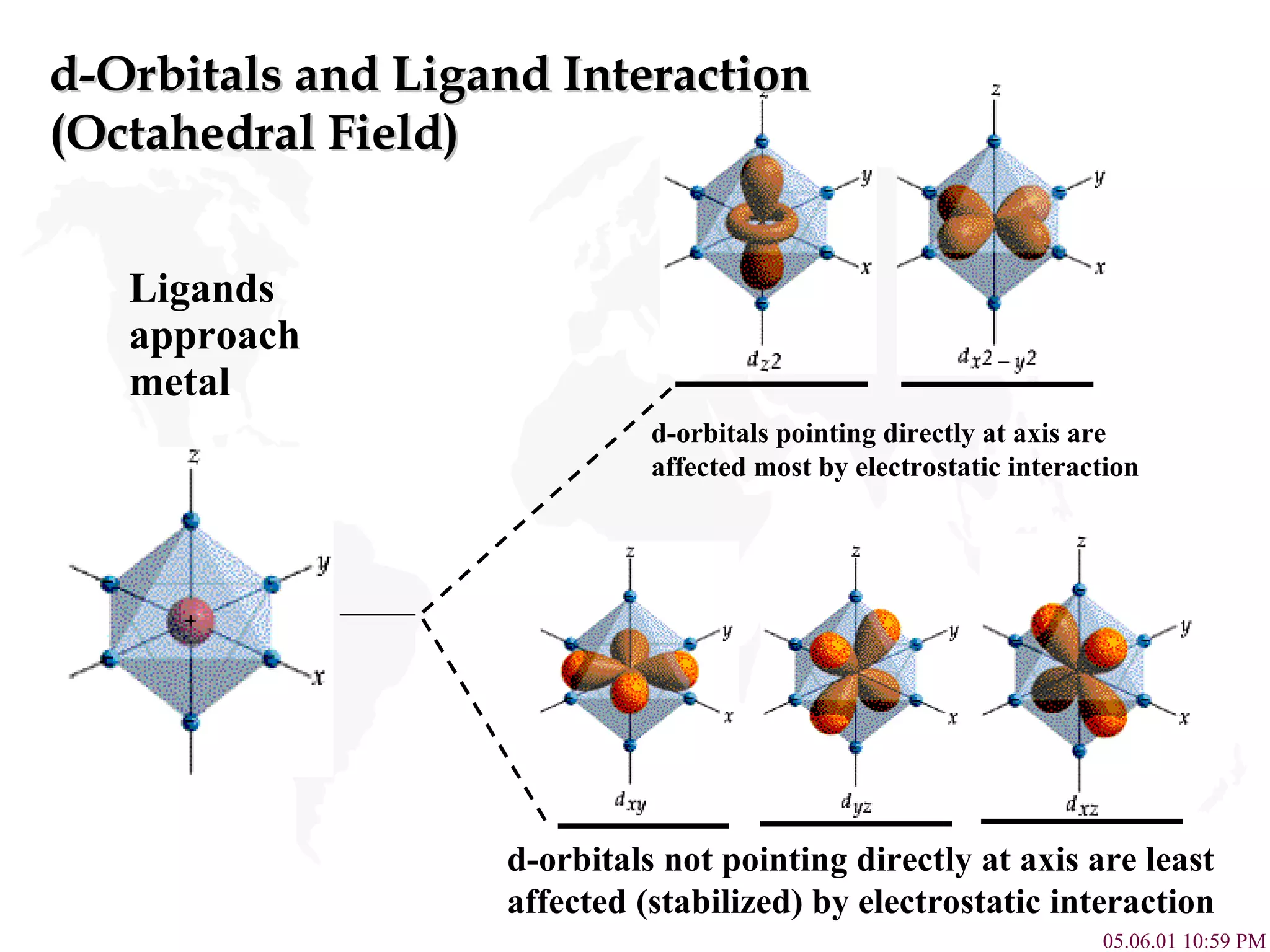
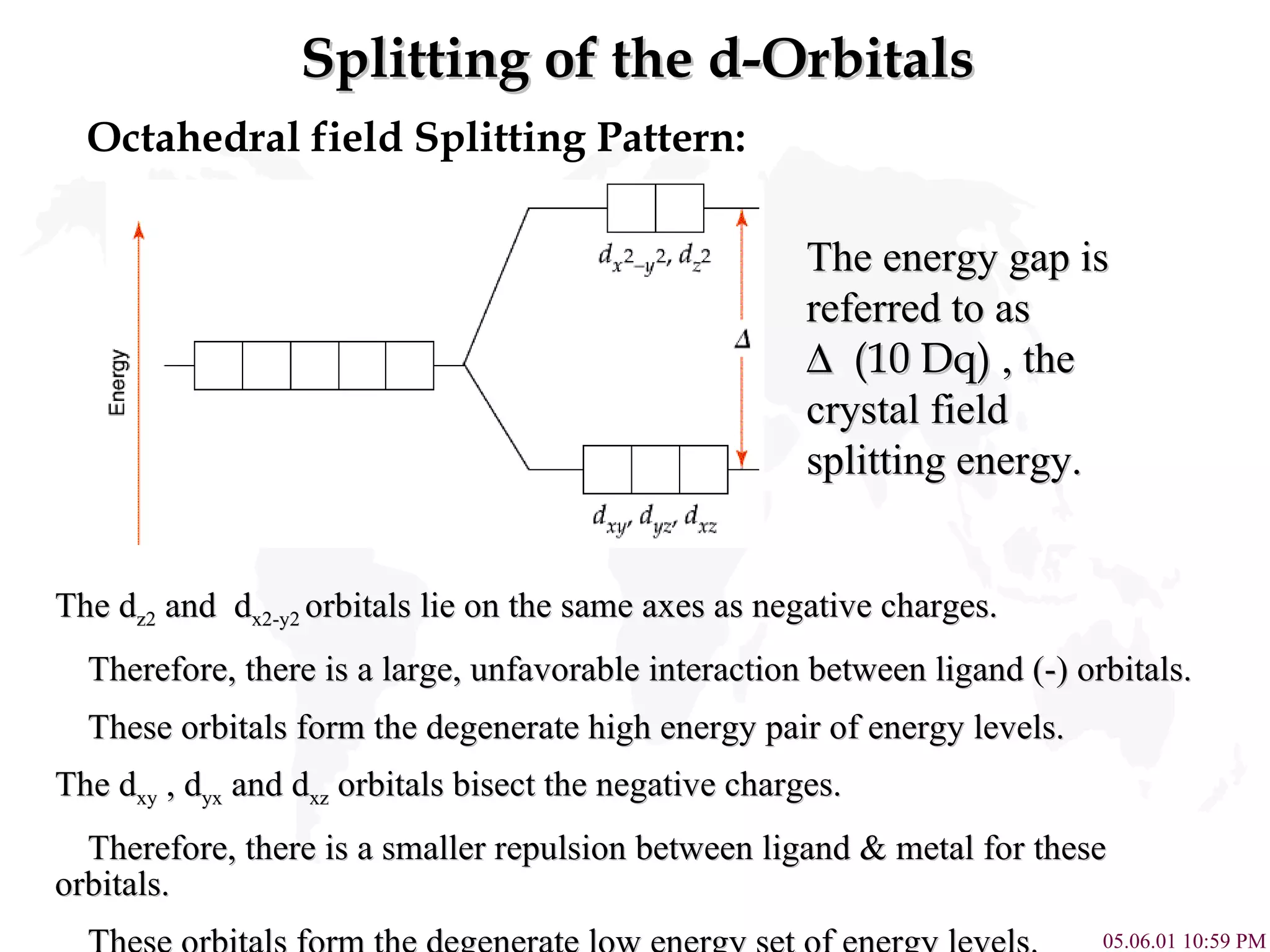
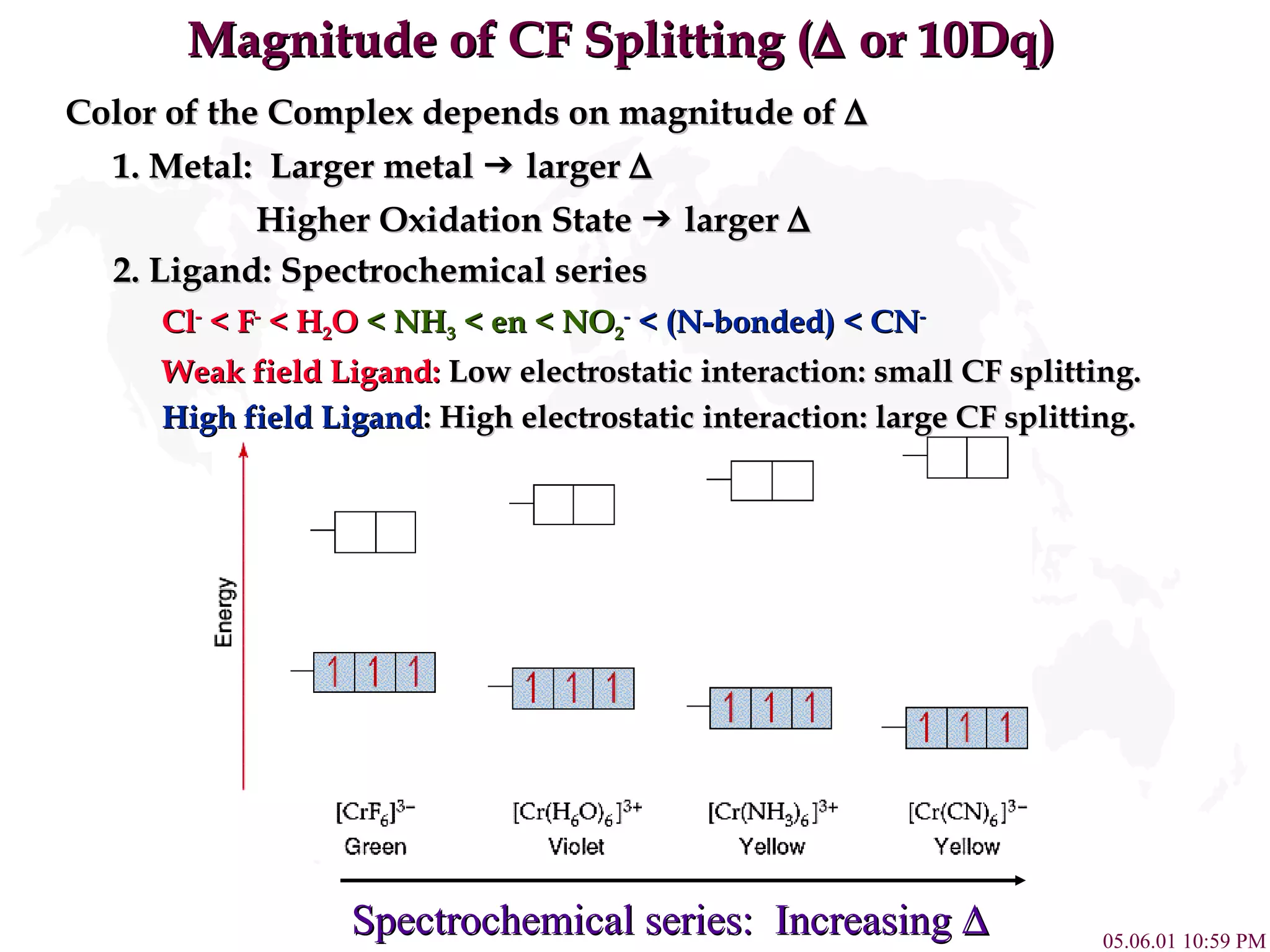
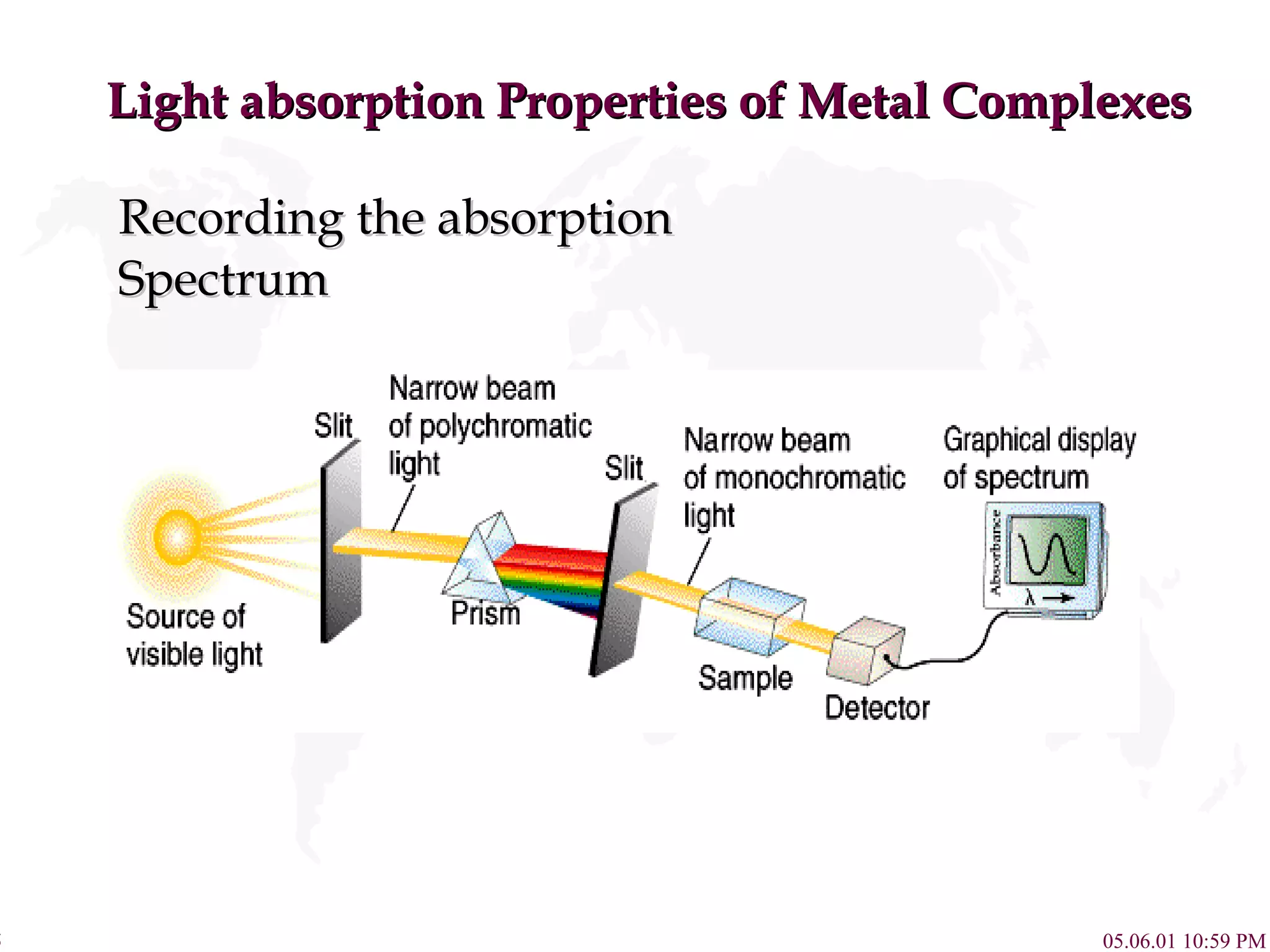
Crystal Field Theory explains the colors of transition metal complexes based on ligand-metal interactions. The electrostatic interaction between ligands and metal d-orbitals splits the d-orbital energies. For an octahedral complex, the d-orbitals point directly at ligands have higher energy than those that bisect ligands. This splitting pattern determines if the complex is high or low spin, which then dictates its color and magnetic properties. The spectrochemical series orders ligands by their ability to cause crystal field splitting, correlating ligand type with complex color.


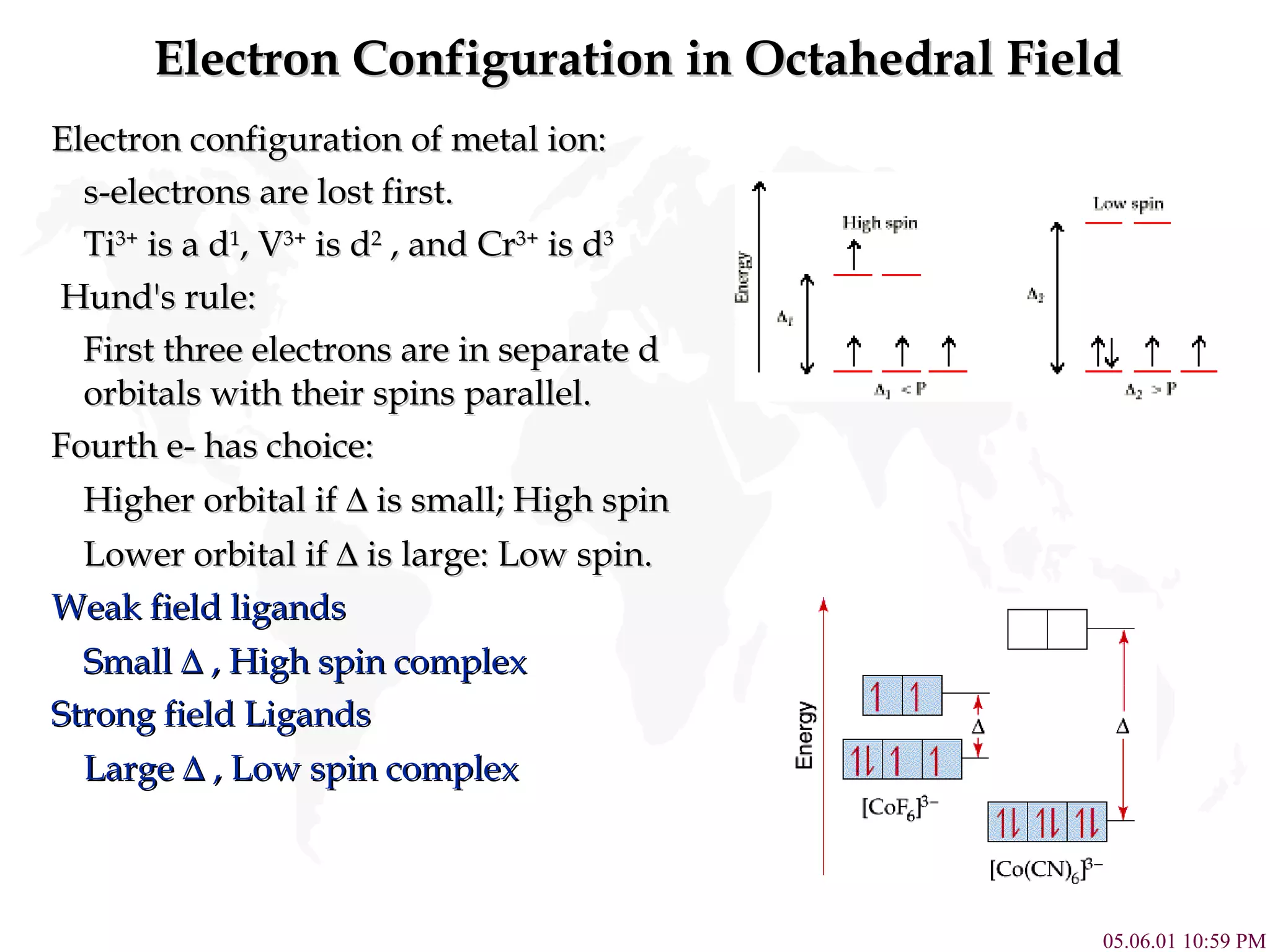
![High Spin Vs. Low Spin (d 1 to d 10 ) Electron Configuration for Octahedral complexes of metal ion having d 1 to d 10 configuration [M(H 2 O) 6 ] +n . Only the d 4 through d 7 cases have both high-spin and low spin configuration . Electron configurations for octahedral complexes of metal ions having from d 1 to d 10 configurations. Only the d 4 through d 7 cases have both high-spin and low-spin configurations.](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/75/Crystal-field-theory-10-2048.jpg)
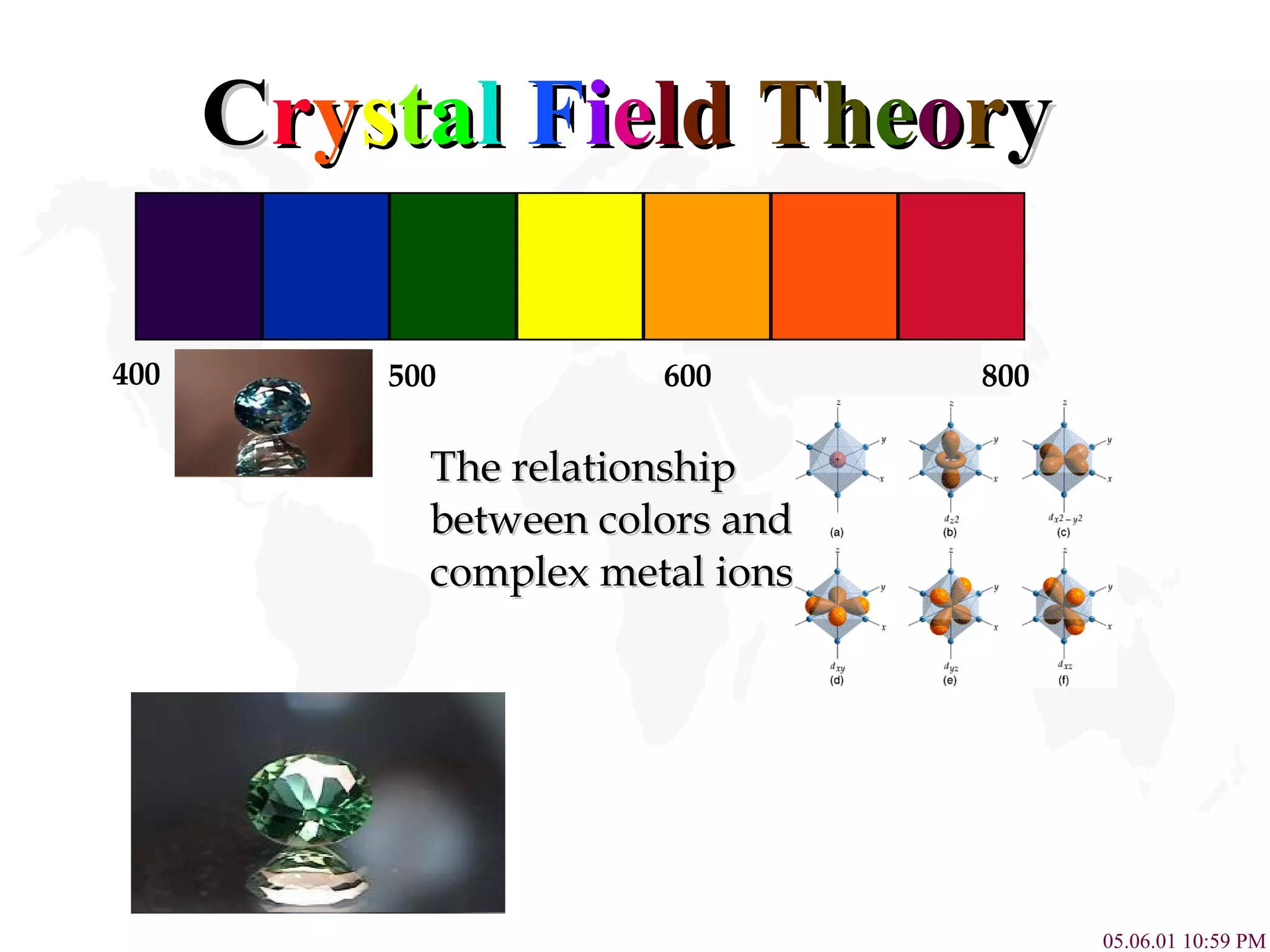
![Color Absorption of Co 3+ Complexes The Colors of Some Complexes of the Co 3+ Ion The complex with fluoride ion, [CoF 6 ] 3+ , is high spin and has one absorption band. The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band. Complex Ion Wavelength of Color of Light Color of Complex light absorbed Absorbed [CoF 6 ] 3+ 700 (nm) Red Green [Co(C 2 O 4 ) 3 ] 3+ 600, 420 Yellow, violet Dark green [Co(H 2 O) 6 ] 3+ 600, 400 Yellow, violet Blue-green [Co(NH 3 ) 6 ] 3+ 475, 340 Blue, violet Yellow-orange [Co(en) 3 ] 3+ 470, 340 Blue, ultraviolet Yellow-orange [Co(CN) 6 ] 3+ 310 Ultraviolet Pale Yellow](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/75/Crystal-field-theory-11-2048.jpg)
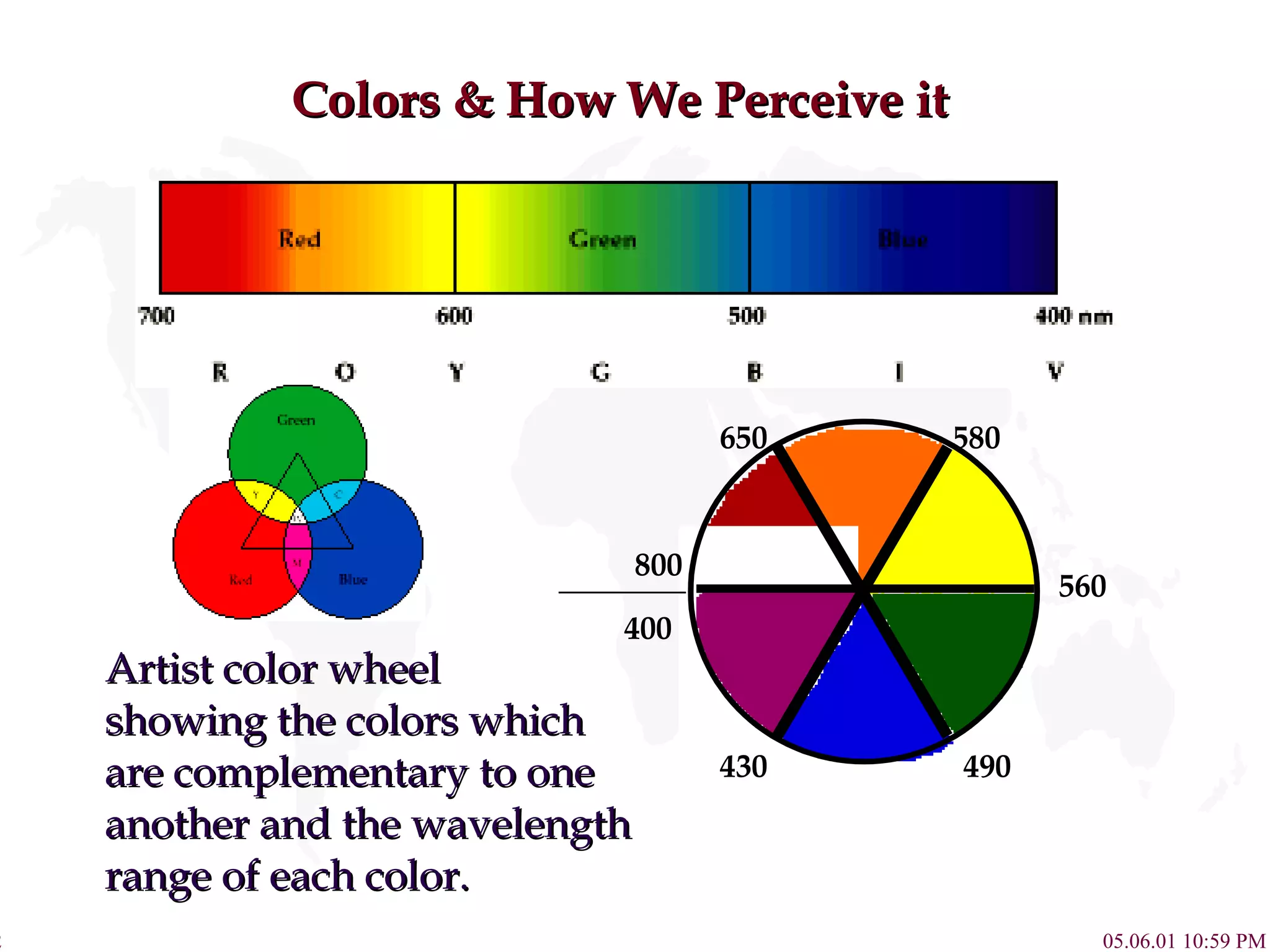
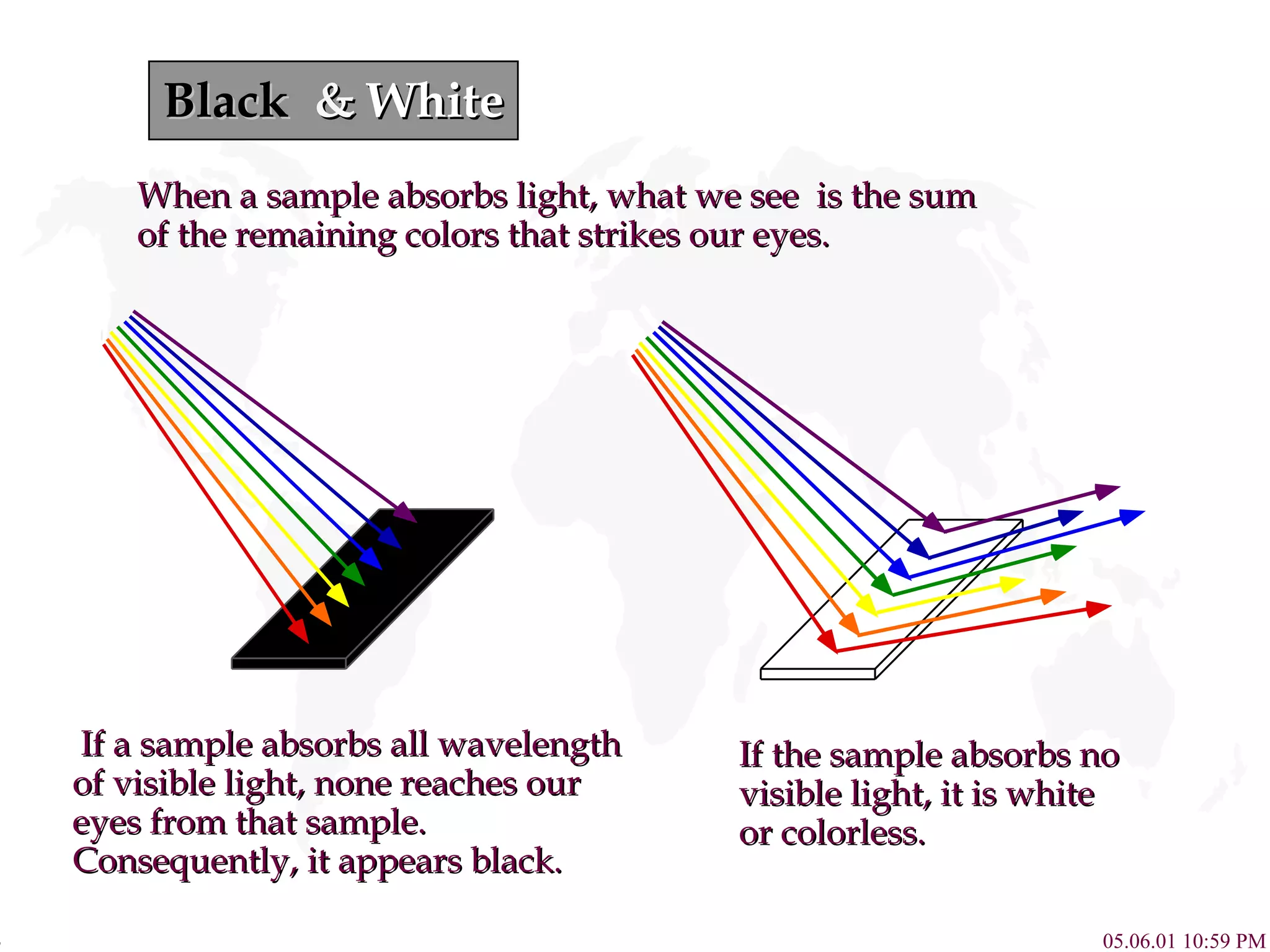
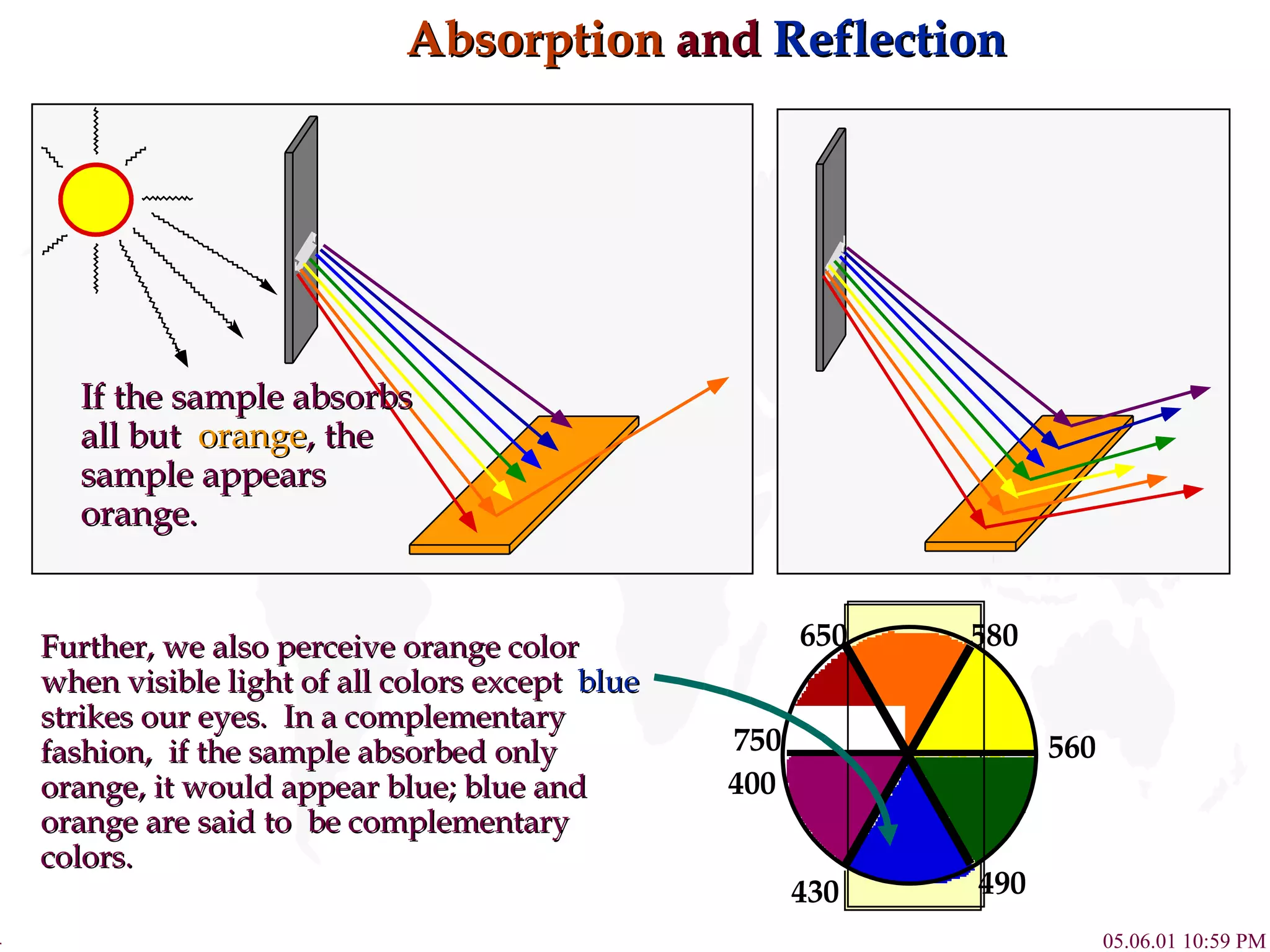
![Complex Influence on Color Compounds of Transition metal complexes solution. [Fe(H 2 O) 6 ] 3+ [Co(H 2 O) 6 ] 2+ [Ni(H 2 O) 6 ] 2+ [Cu(H 2 O) 6 ] 2+ [Zn(H 2 O) 6 ] 2+ 800 430 650 580 560 490 400](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/75/Crystal-field-theory-16-2048.jpg)
![Color Absorption of Co 3+ Complexes The Colors of Some Complexes of the Co 3+ Ion The complex with fluoride ion, [CoF 6 ] 3+ , is high spin and has one absorption band. The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band.](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/75/Crystal-field-theory-17-2048.jpg)