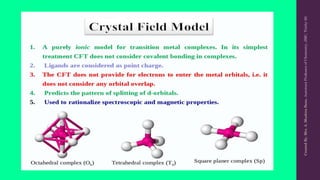
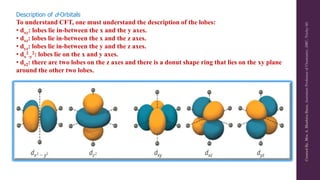
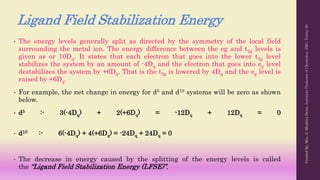
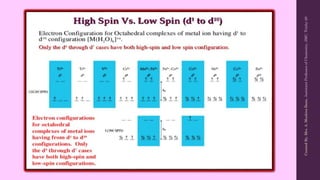
Crystal Field Theory (CFT) explains the splitting of d-orbital energy levels in octahedral transition metal complexes due to ligand interactions. The d-orbitals split into higher energy (eg) and lower energy (t2g) levels, impacting magnetic properties and color. The energy difference between these levels is known as ligand field stabilization energy (LFSE), which contributes to the system's stability.