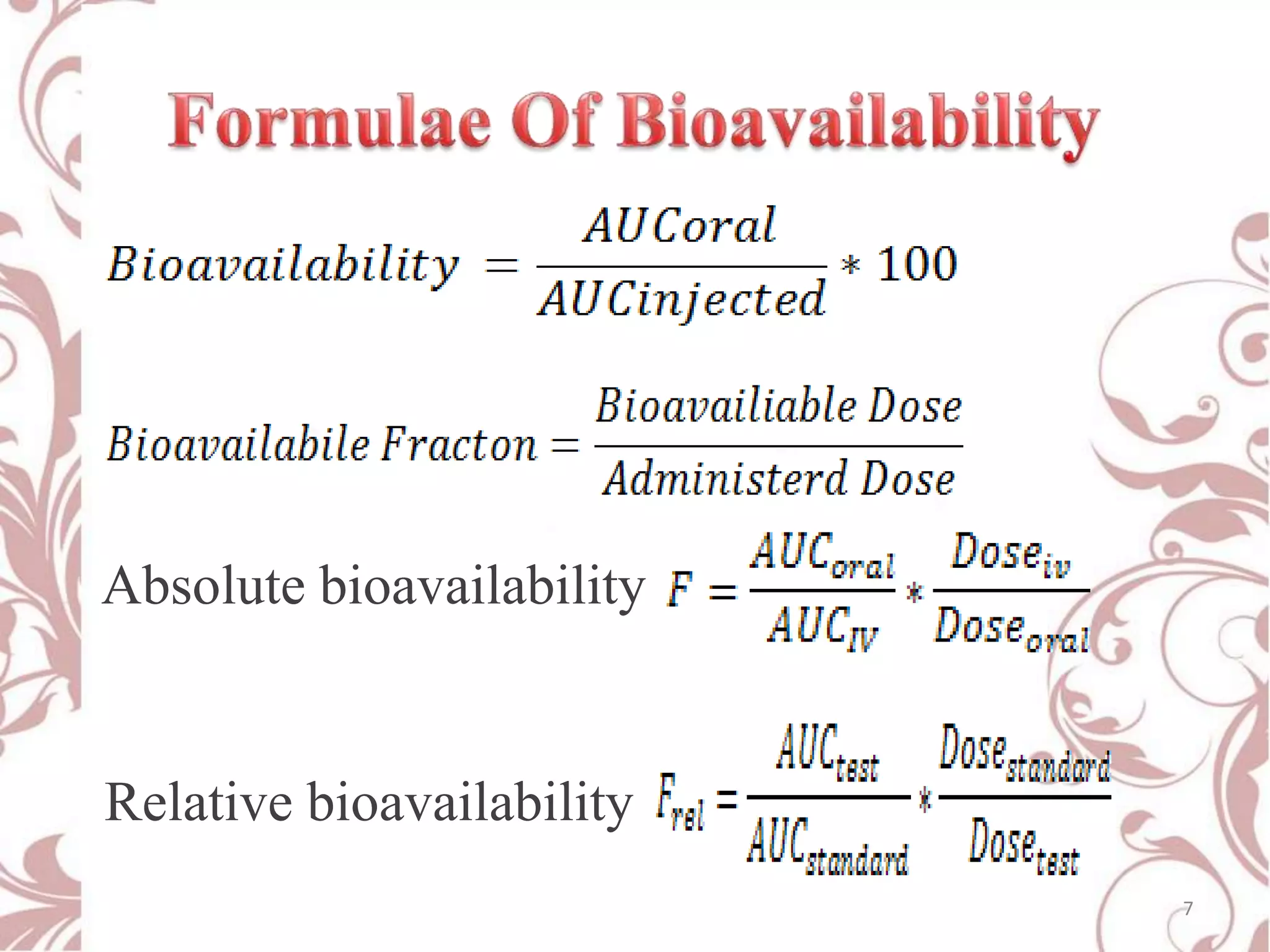
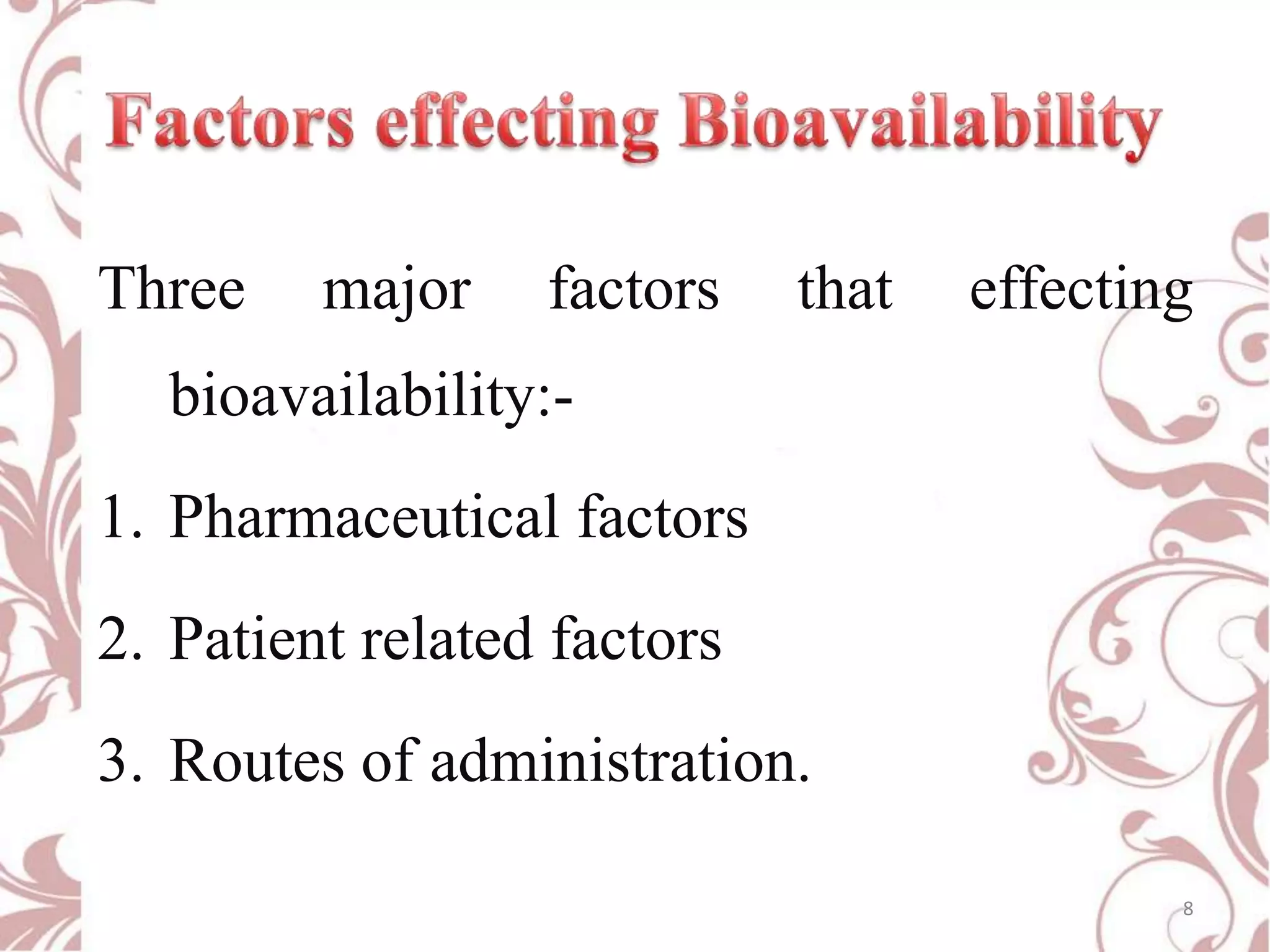
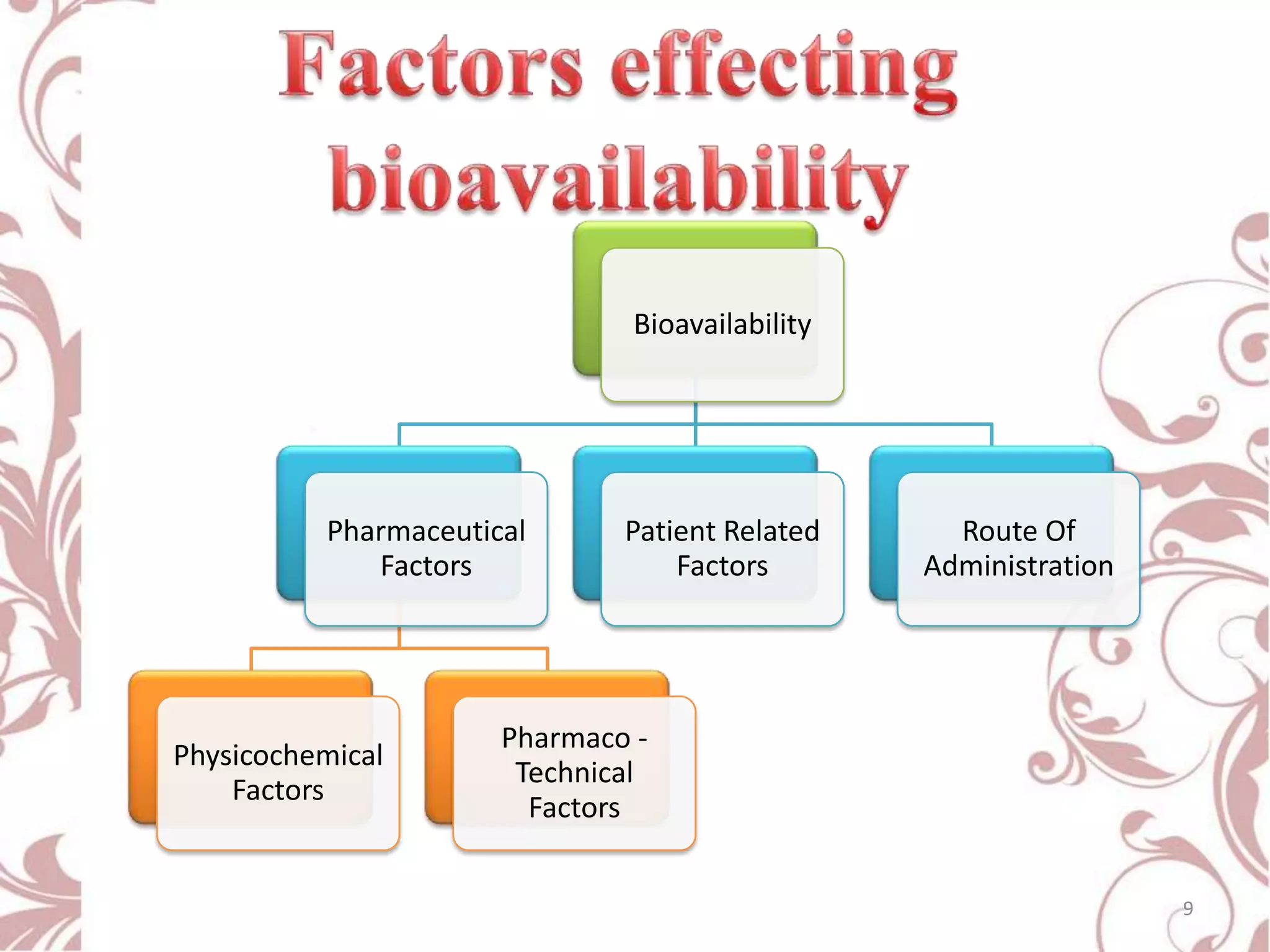
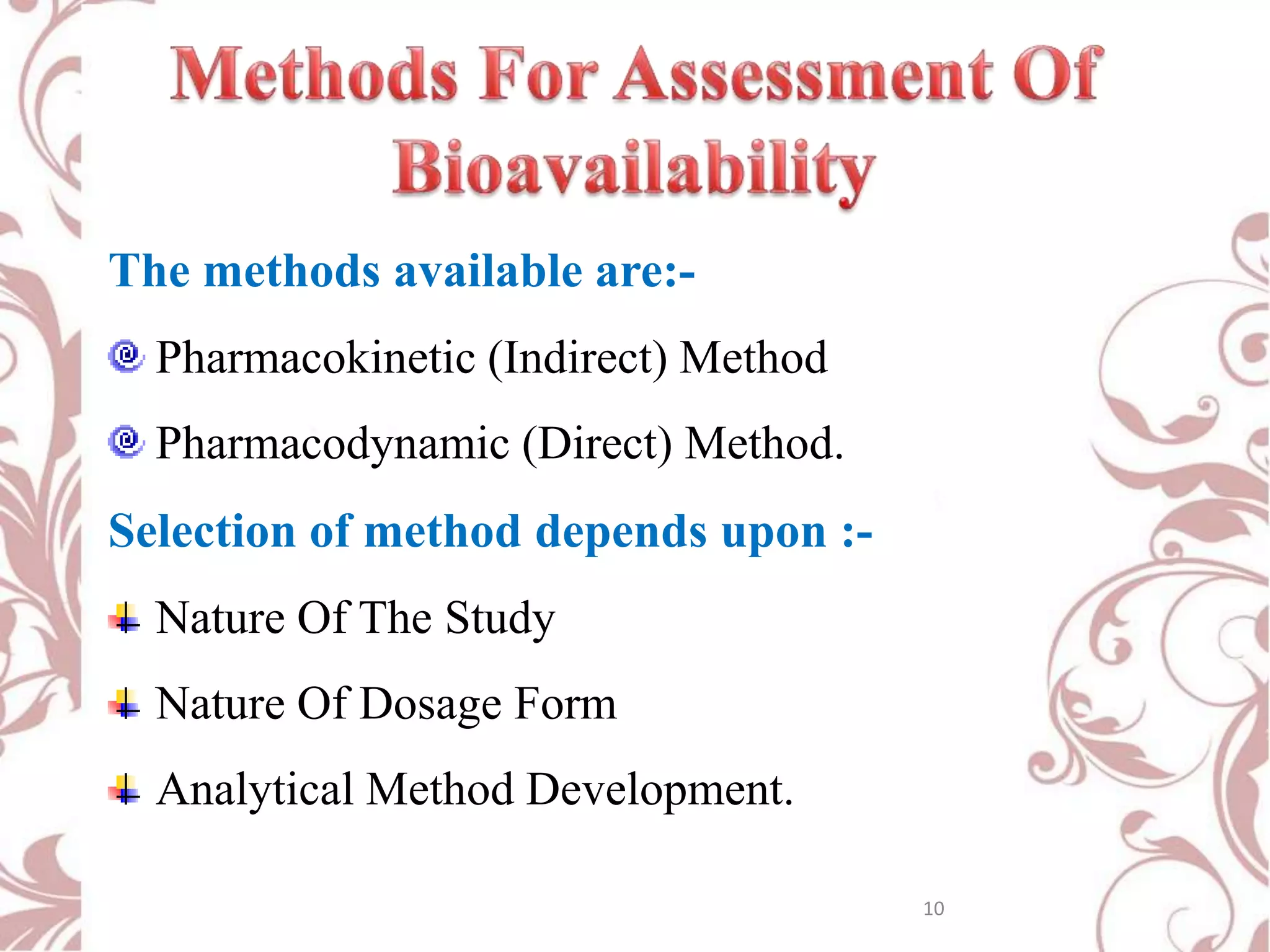
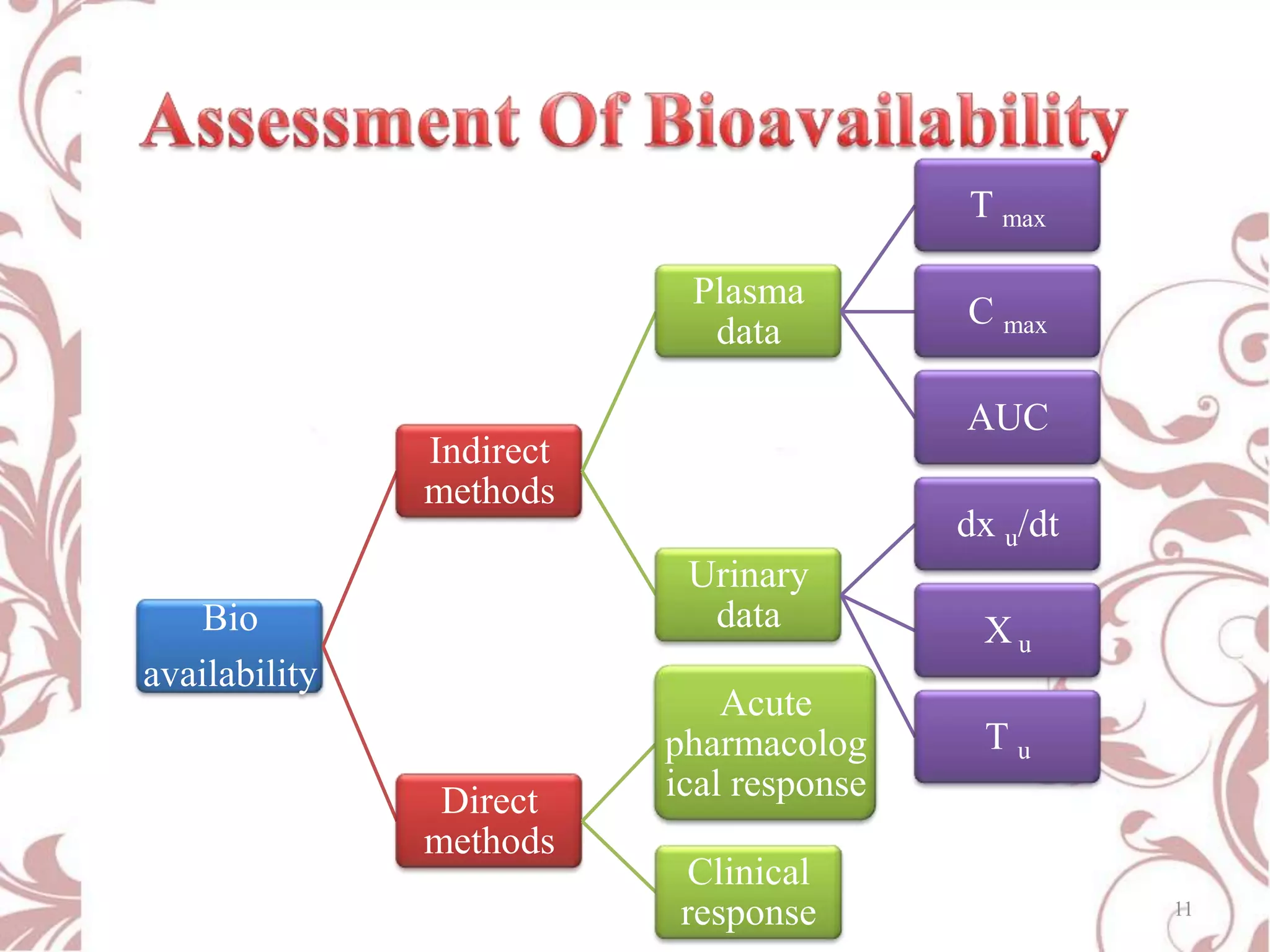
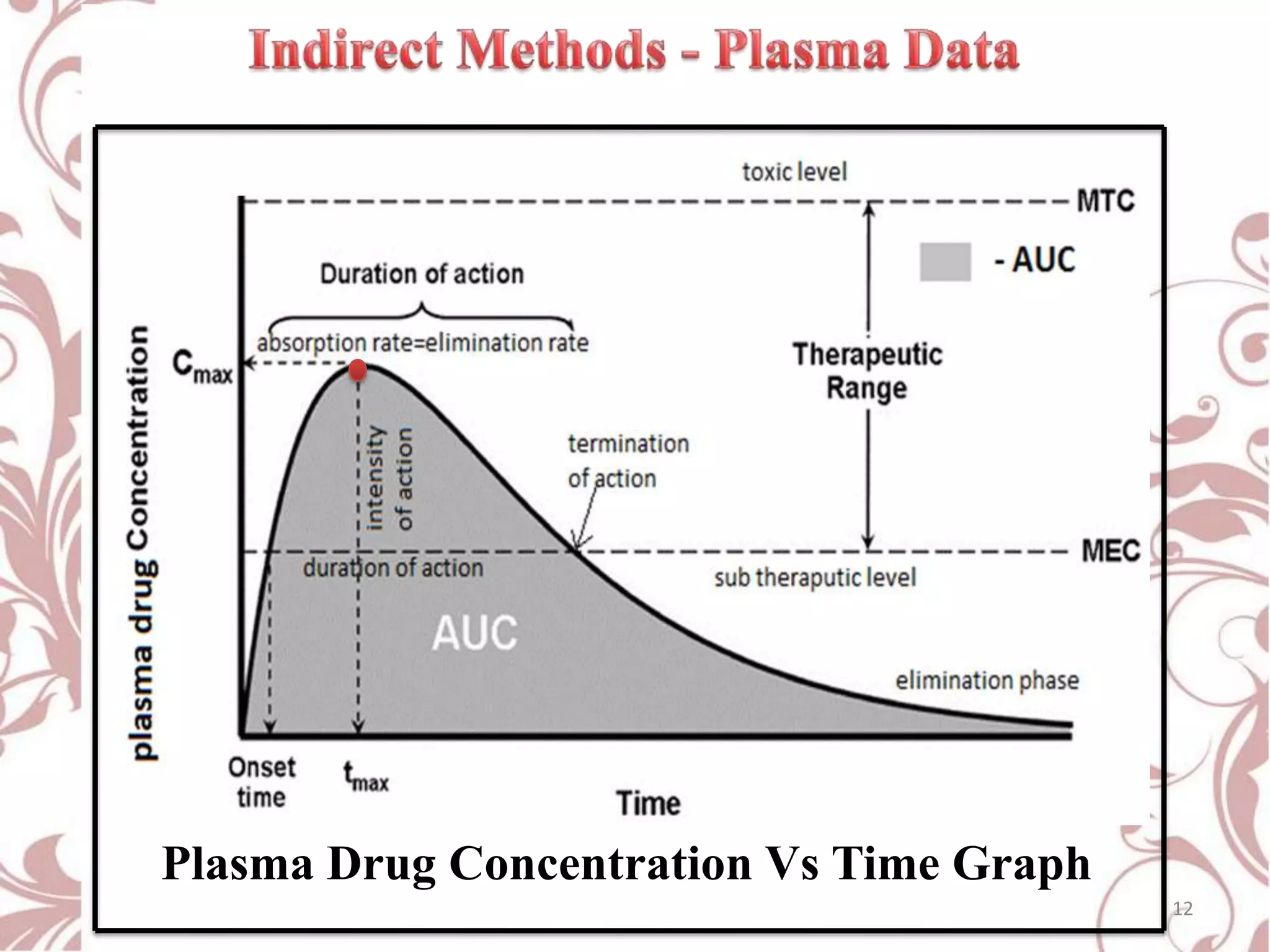
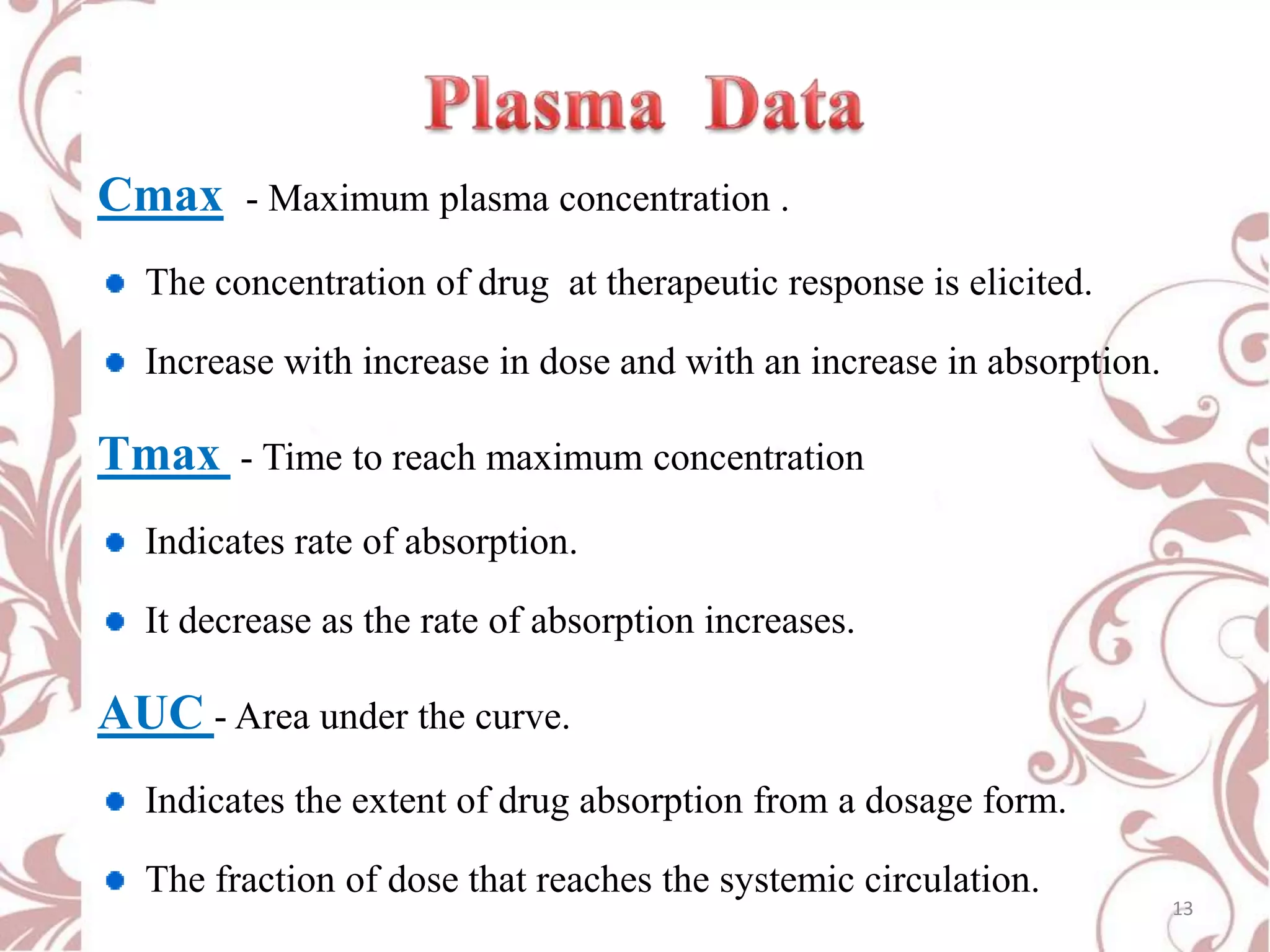
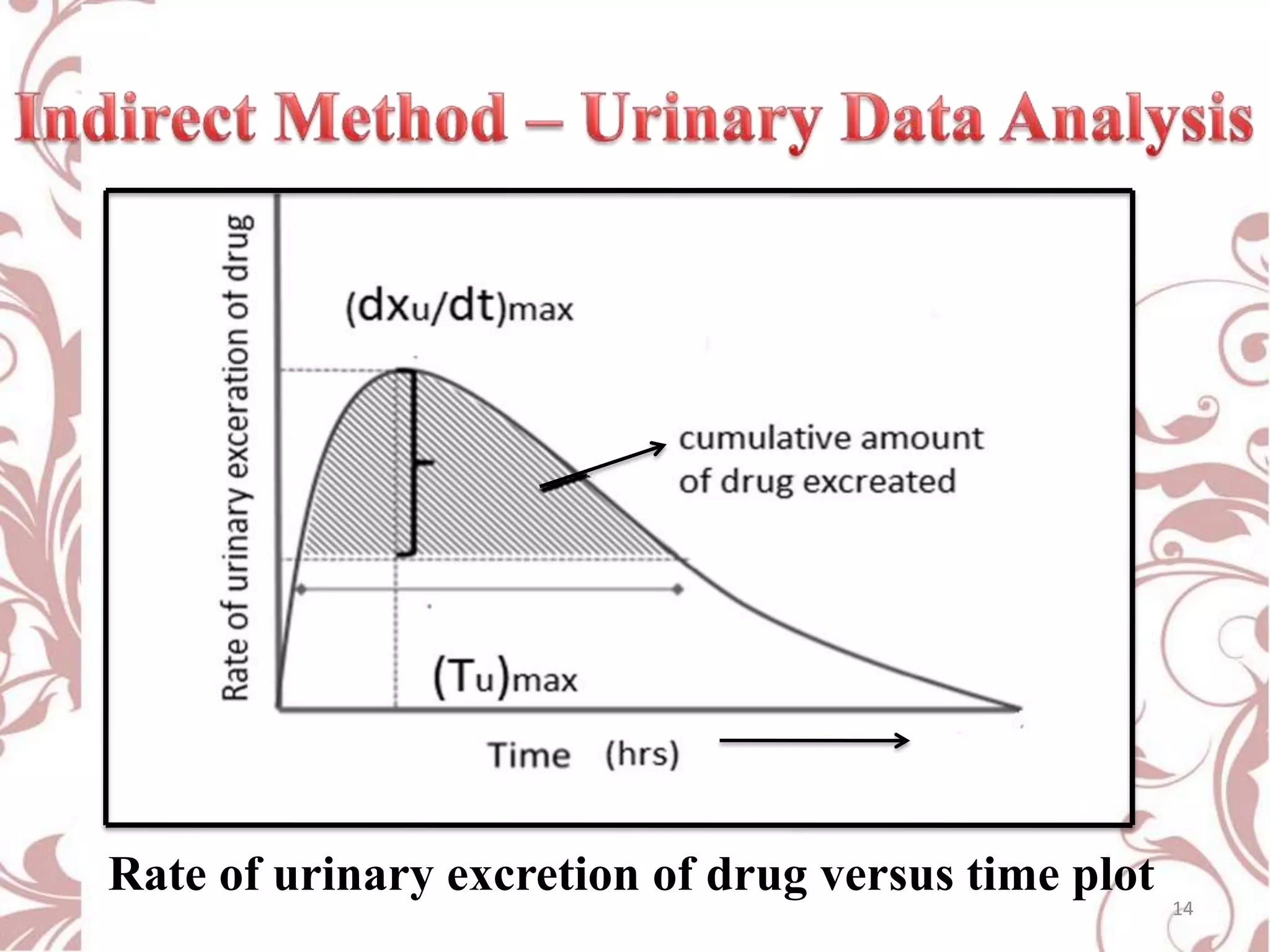
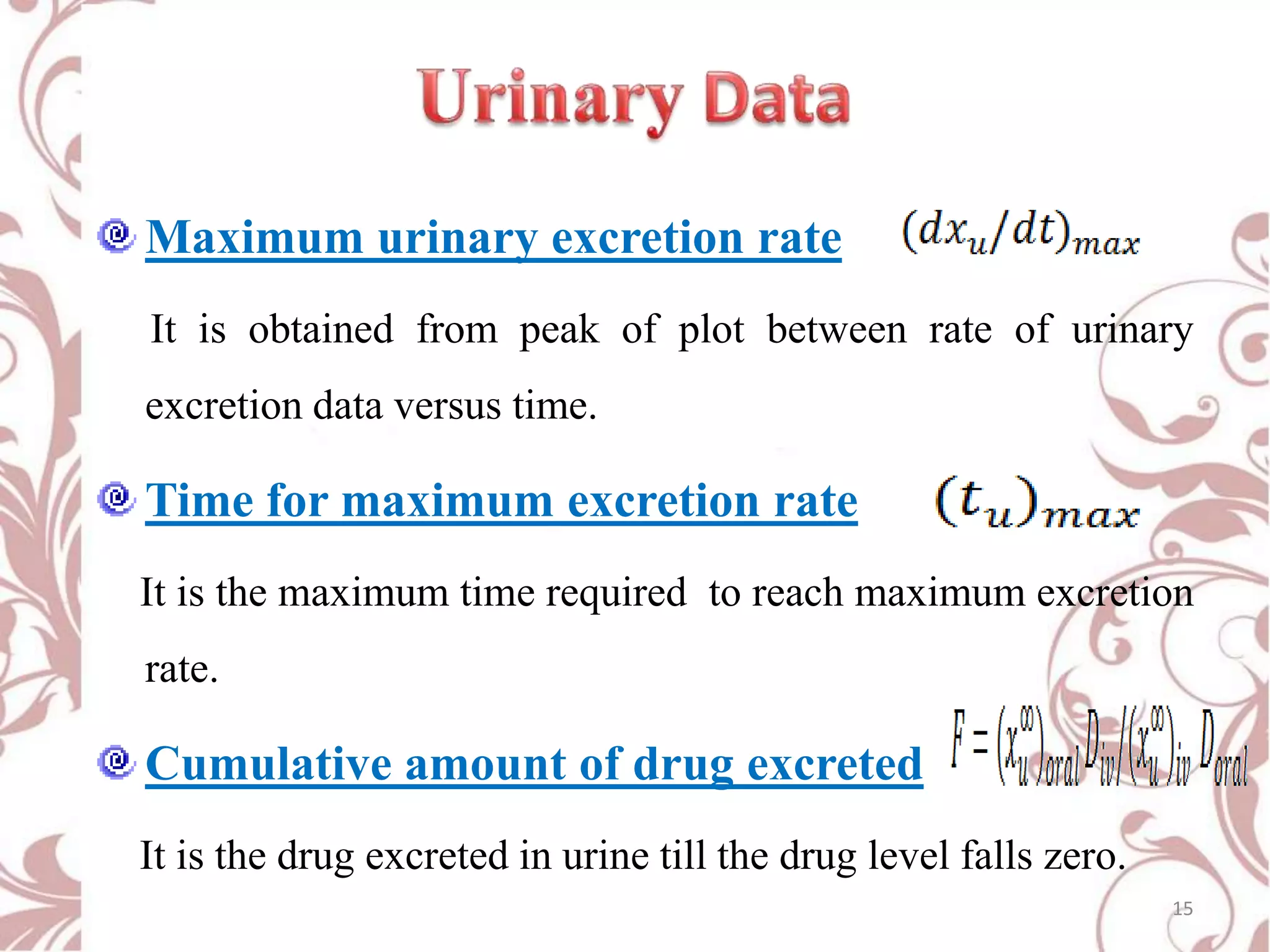
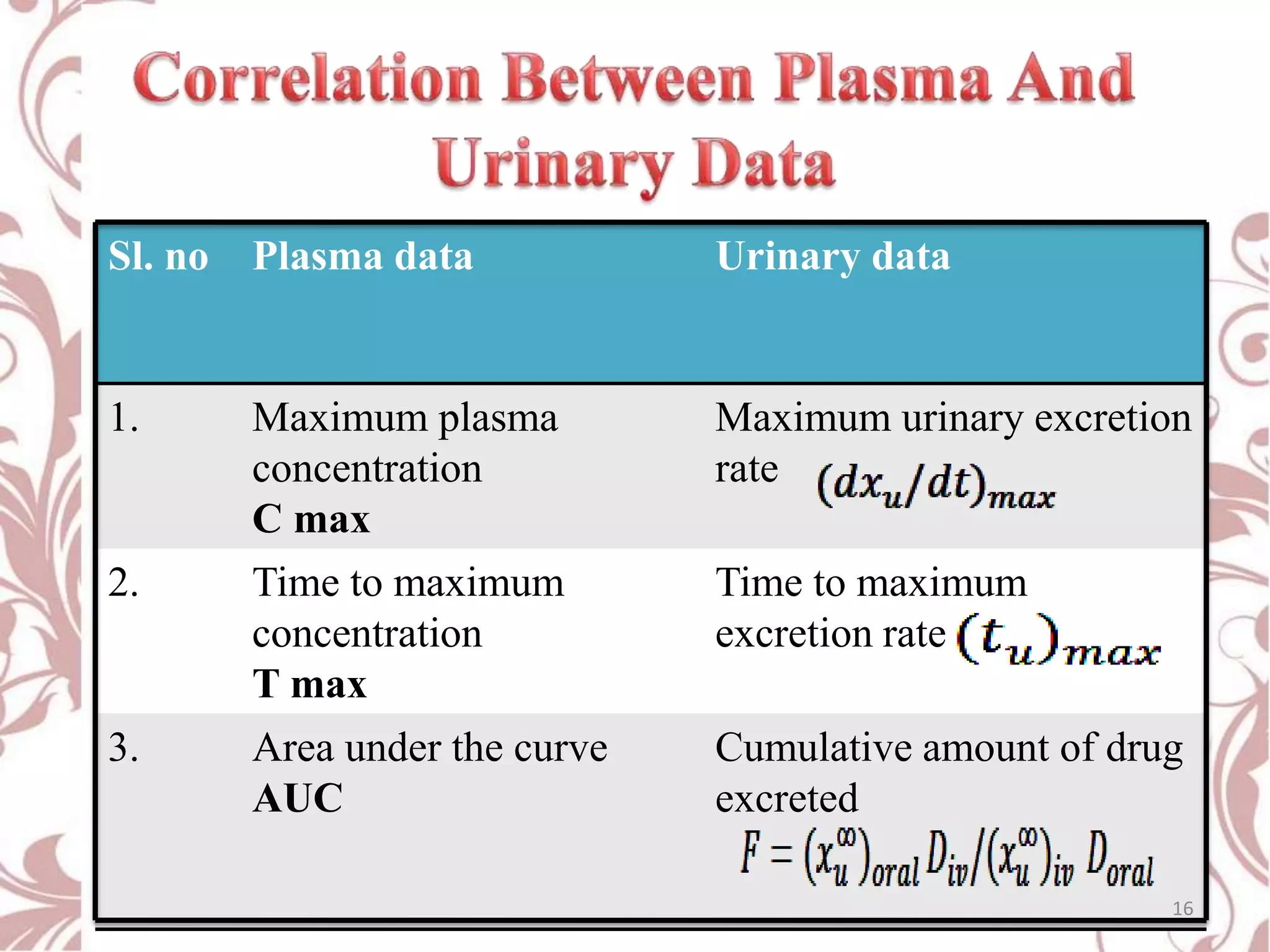
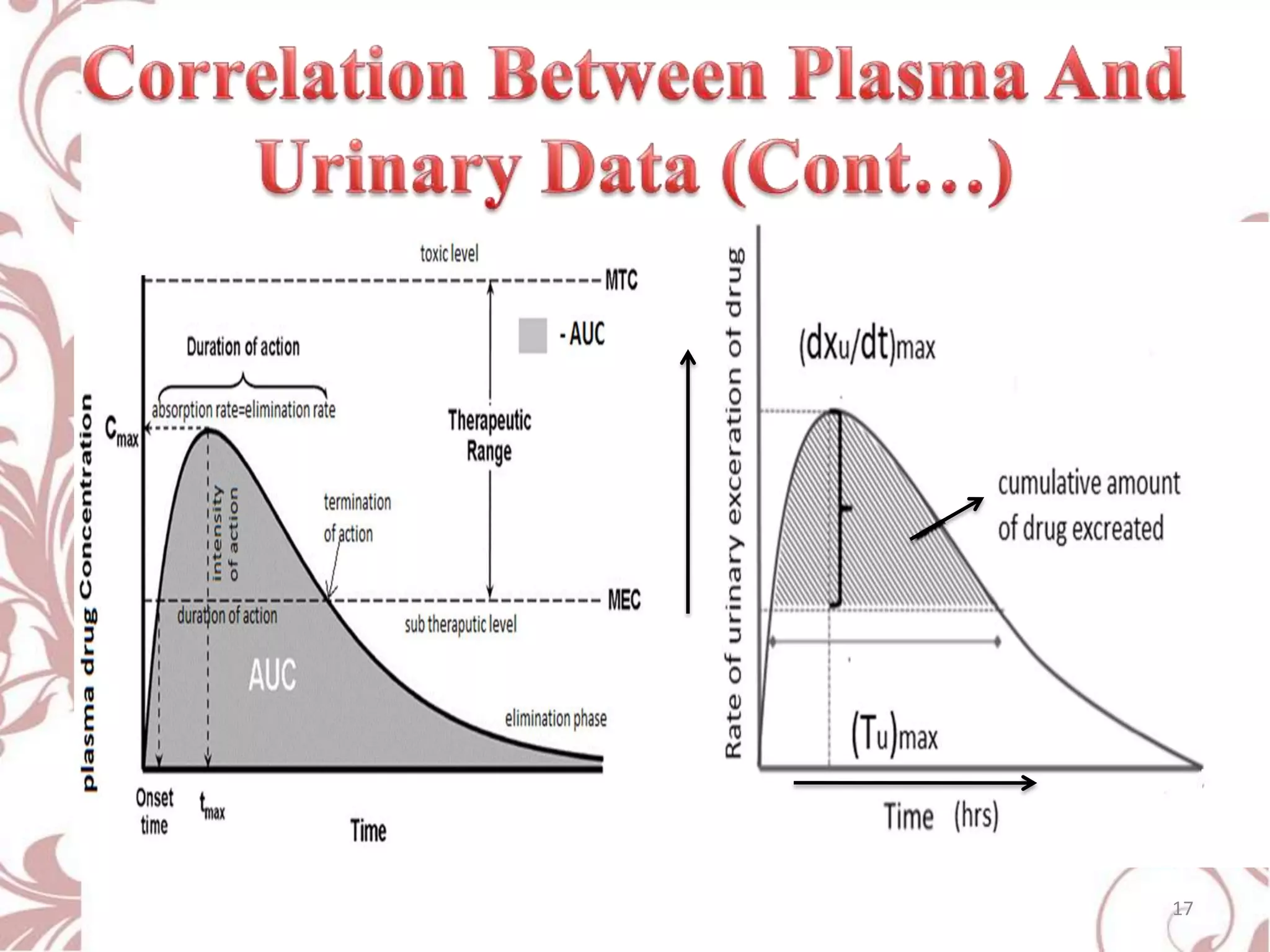
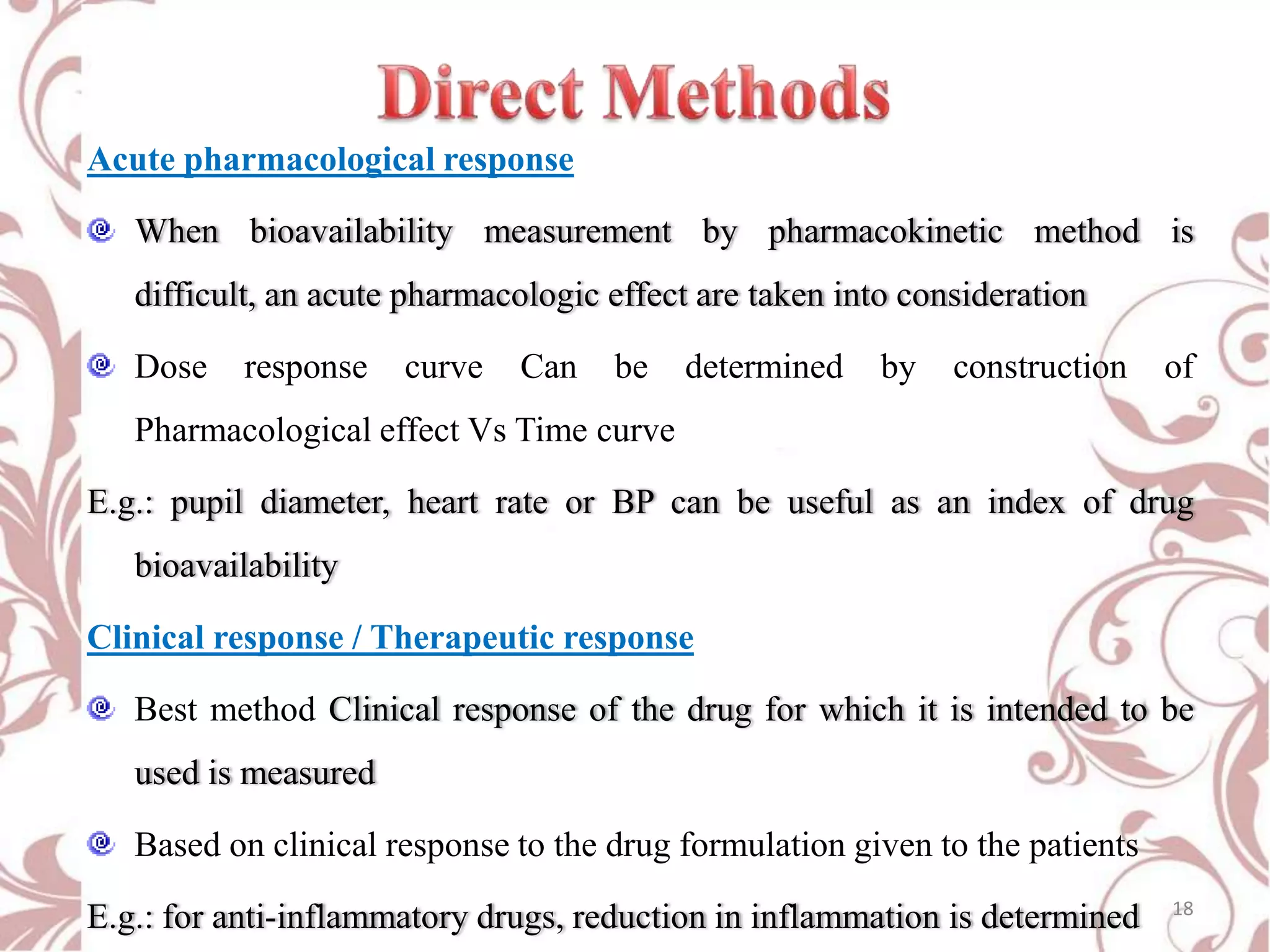
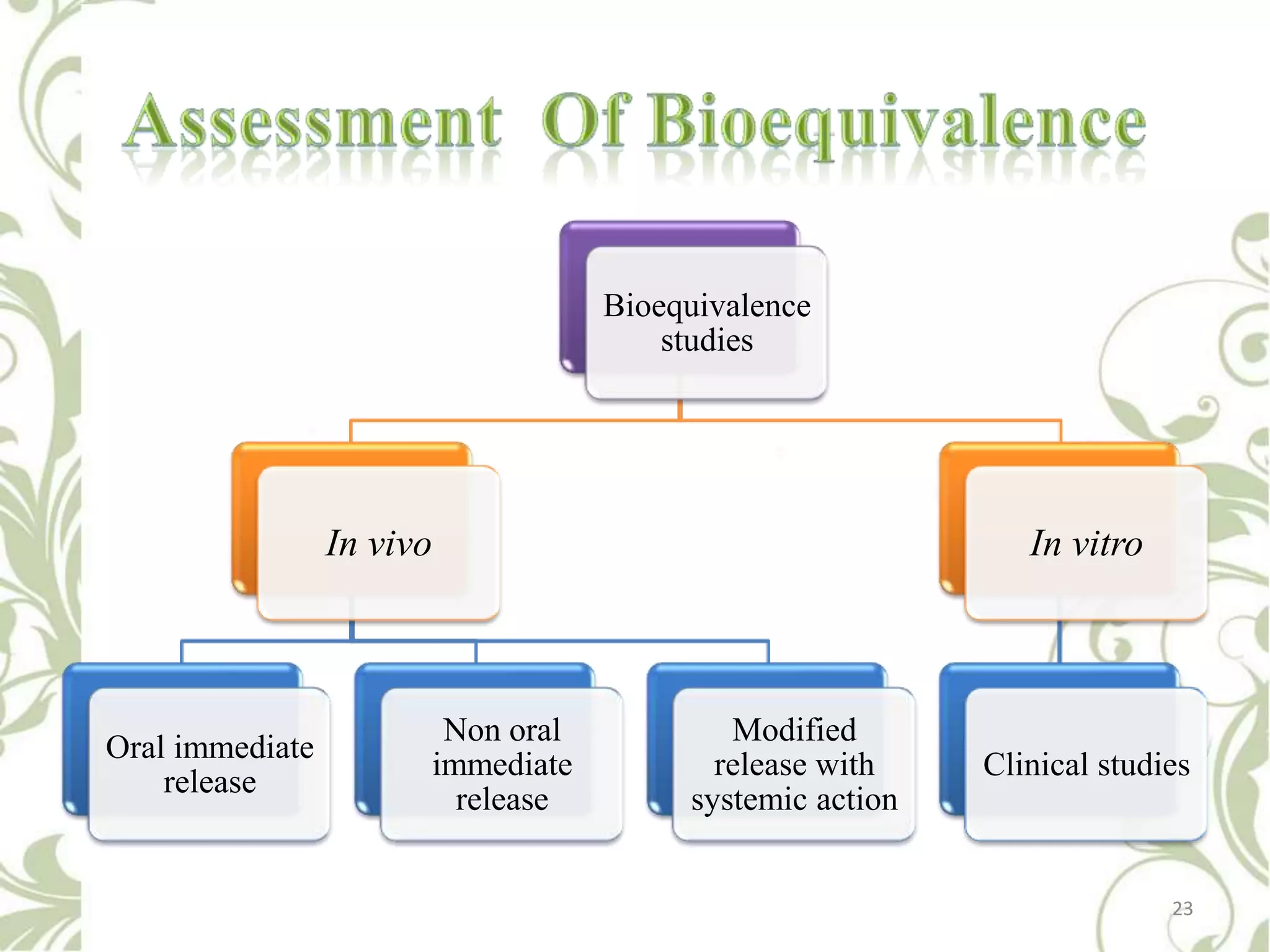
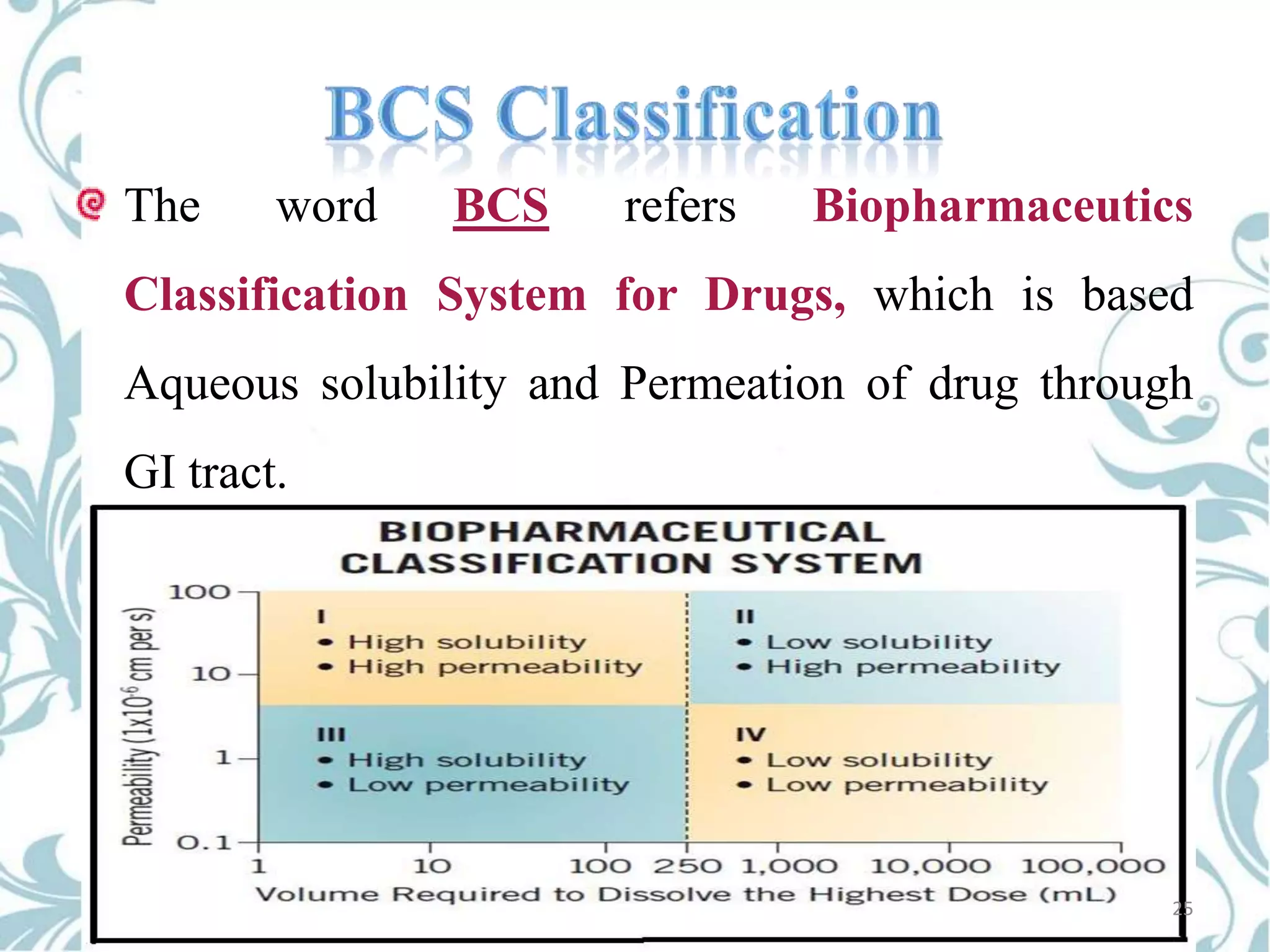
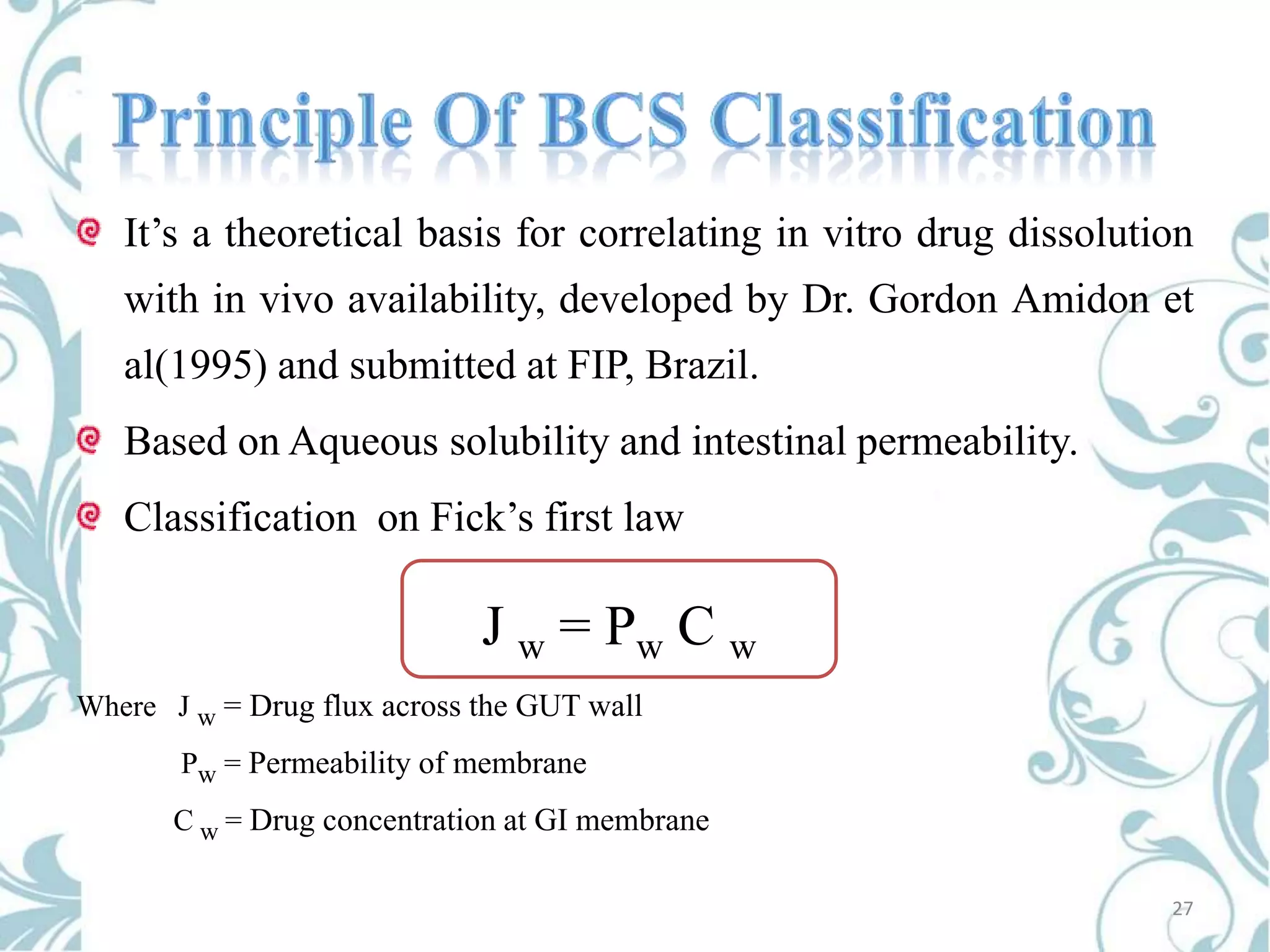
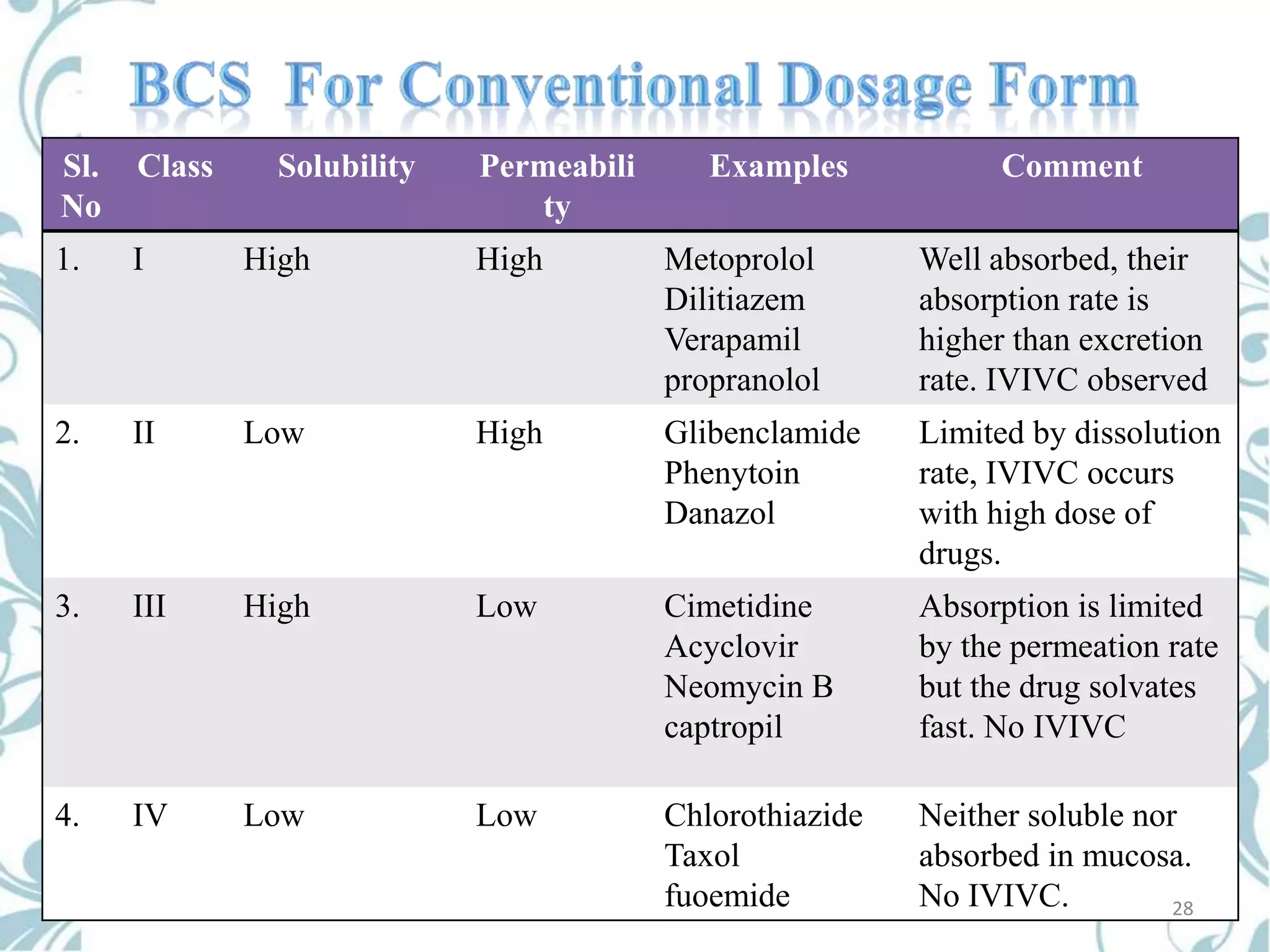
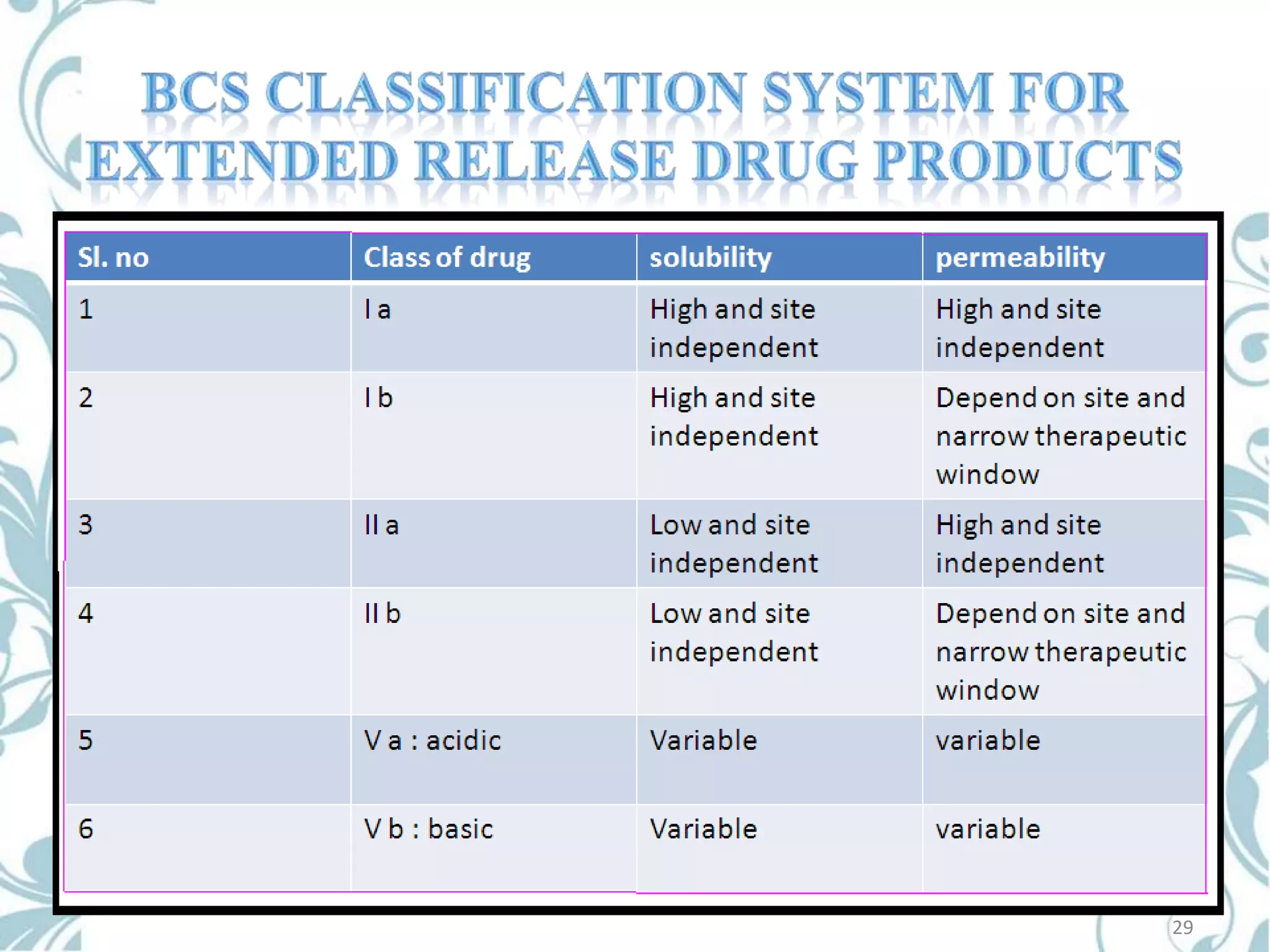
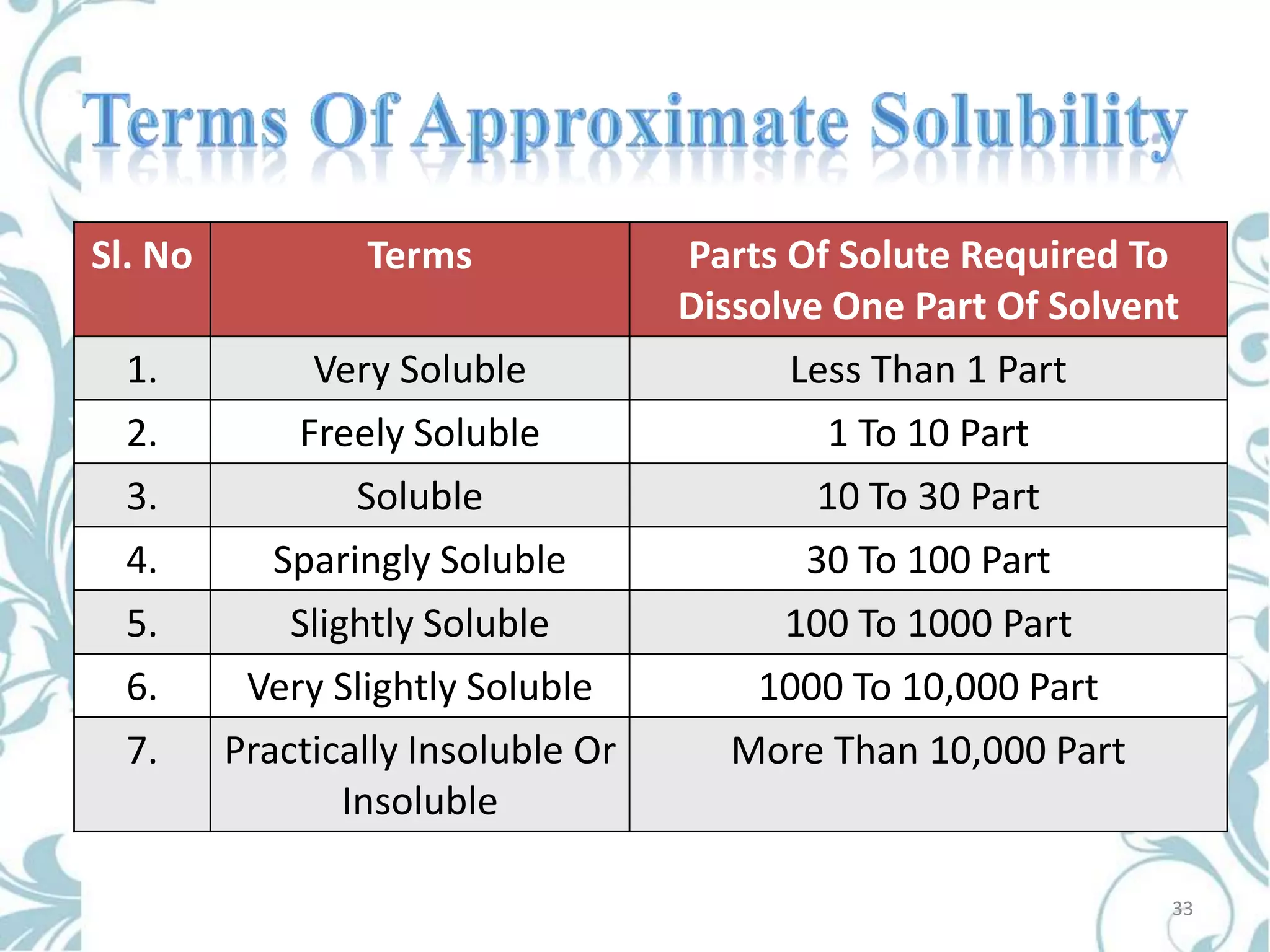
This document provides an overview of bioavailability, bioequivalence, and BCS classification. It defines key terms like bioavailability, absolute and relative bioavailability, and factors affecting bioavailability. Measurement methods like pharmacokinetic and pharmacodynamic approaches are discussed. Bioequivalence studies and their types are summarized. The six classes of BCS classification and its applications in drug development are highlighted in brief. In conclusion, the document notes that BCS classification provides guidance in drug formulation and review processes to reduce costs and time of approval.