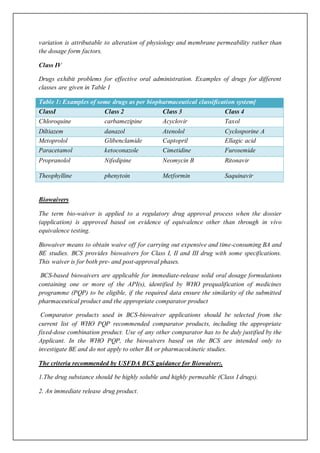
The Biopharmaceutics Classification System (BCS) classifies drug substances based on their aqueous solubility and intestinal permeability. It includes four classes of drugs. Class 1 drugs are highly soluble and highly permeable. Class 2 are low solubility and highly permeable. Class 3 are highly soluble and low permeability. Class 4 are low solubility and low permeability.
The BCS is used to determine if in vivo bioequivalence studies can be waived for immediate release oral drugs. Class 1 drugs that are rapidly dissolving may not need these studies if dissolution profiles between brands are similar. This can reduce costs for approval. The criteria for classifying solubility and permeability and determining dissolution are defined in regulatory guidance documents.




![Acyclovir
Foscarnet
Mannitol
Chlorothiazide
Zero Permeability Inulin
Lactulose
Efflux Substrates Digoxin
Quinidine
Vinblastine
3. Dissolution
According to USFDA BCS guidance an IR drug product is considered rapidly dissolving
when no less than 85% of the labeled amount of the drug substance dissolves within 30
minutes, using USP apparatus I at 100 rpm (or Apparatus II at 50 rpm) in a volume of 900
ml or less in each medium: 0.1 N HCl or simulated gastric fluid USP without enzymes; buffer
(pH 4.5); and buffer (pH 6.8) or simulated intestinal fluid USP without enzymes. According
to WHO BCS guidance a multisource product (pharmaceutically equivalent or
pharmaceutically alternative products that may or may not be therapeutically equivalent) is
considered to be very rapidly dissolving when no less than 85% of the labeled amount of the
drug substance dissolves in 15 minutes using a paddle apparatus at 75 rpm or a basket
apparatus at 100 rpm in a volume of 900 ml or less in each medium: HCl solution (pH 1.2);
acetate buffer (pH 4.5); and phosphate buffer (pH 6.8). A multisource product is considered
to be rapidly dissolving when no less than 85% of the labeled amount of the drug substance
dissolves in 30 minutes using a paddle apparatus at 75 rpm or a basket apparatus at 100 rpm
in a volume of 900 ml or less in each of the media: HCl solution (pH 1.2); acetate buffer (pH
4.5); and phosphate buffer (pH 6.8).
A minimum of 12 dosage units of a drug product should be evaluated to support a biowaiver
request. Samples should be collected at a sufficient number of intervals to characterize the
dissolution profile of the drug product (e.g., 5, 10, 15, 20, and 30 minutes). When comparing the
test and reference products, dissolution profiles should be compared 320 using a similarity factor
(f2).
f2 = 50 • log {[1 + (1/n)Σt=1n (Rt - Tt)2]-0.5 • 100}
The similarity factor is a logarithmic reciprocal square root transformation of the sum of
squared error and is a measurement of the similarity in the percent (%) of dissolution
between the two curves;
n is the number of time points
Rt is the dissolution value of the reference batch at time t
Tt is the dissolution value of the test batch at time t.
Two dissolution profiles are considered similar when the f2 value is ≥50. To allow the use of
mean data, the coefficient of variation should not be more than 20 percent at the earlier time
points (e.g., 10 minutes), and should not be more than 10 percent at other time points. Note
that when both test and reference products dissolve 85 percent or more of the label amount of](https://image.slidesharecdn.com/thebiopharmaceuticsclassificationsystem-180409065758/85/The-biopharmaceutics-classification-system-5-320.jpg)