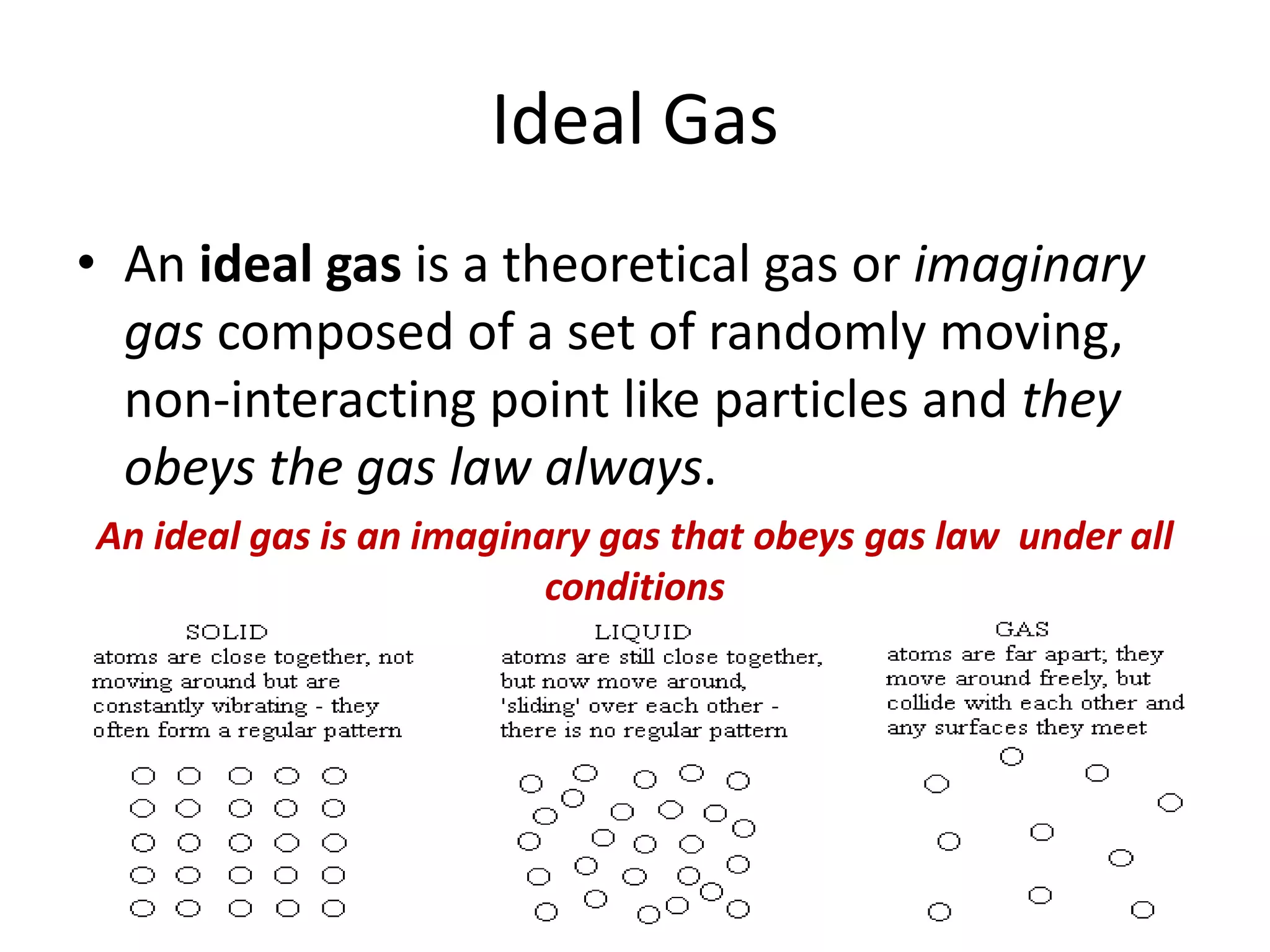
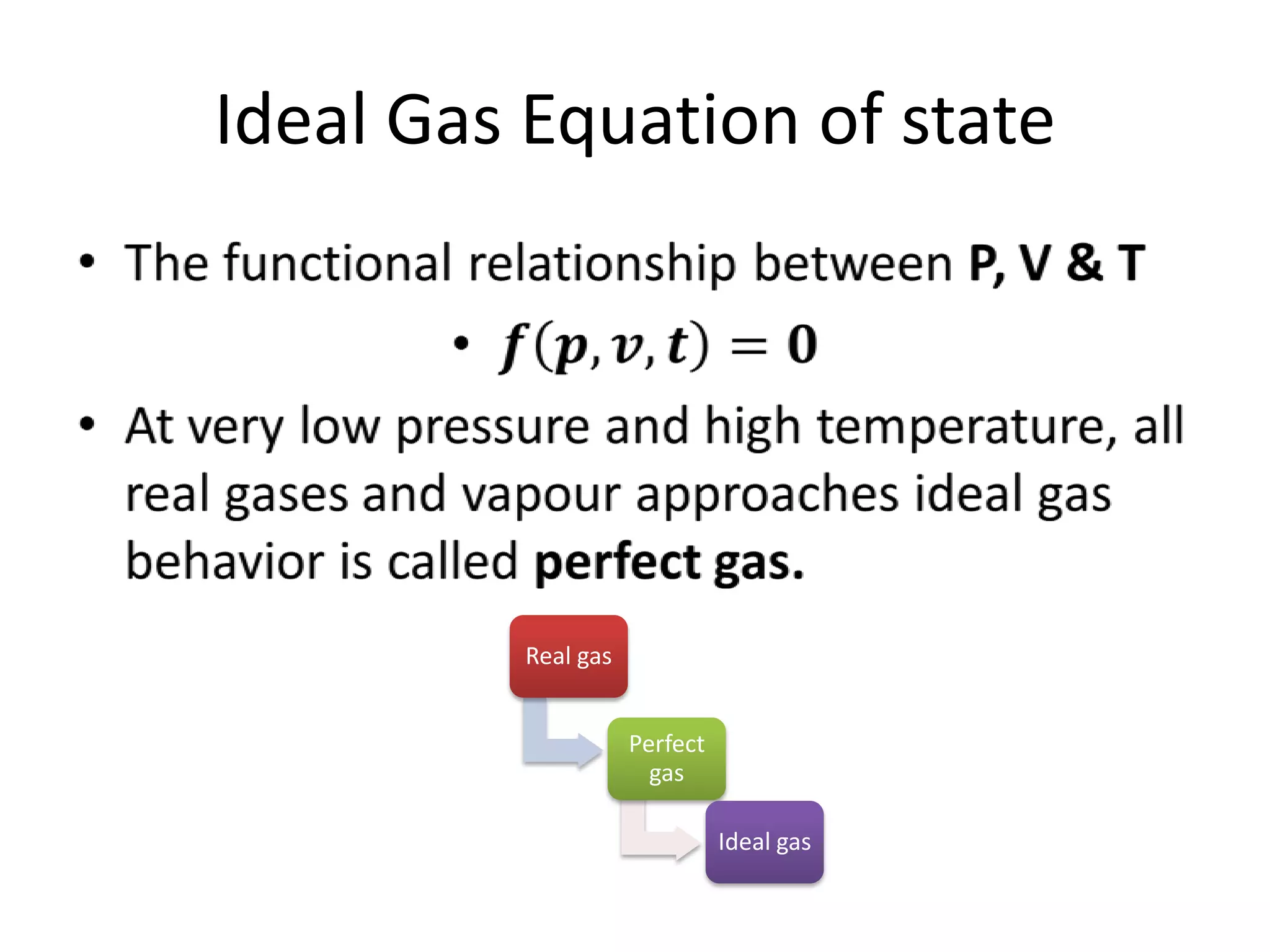
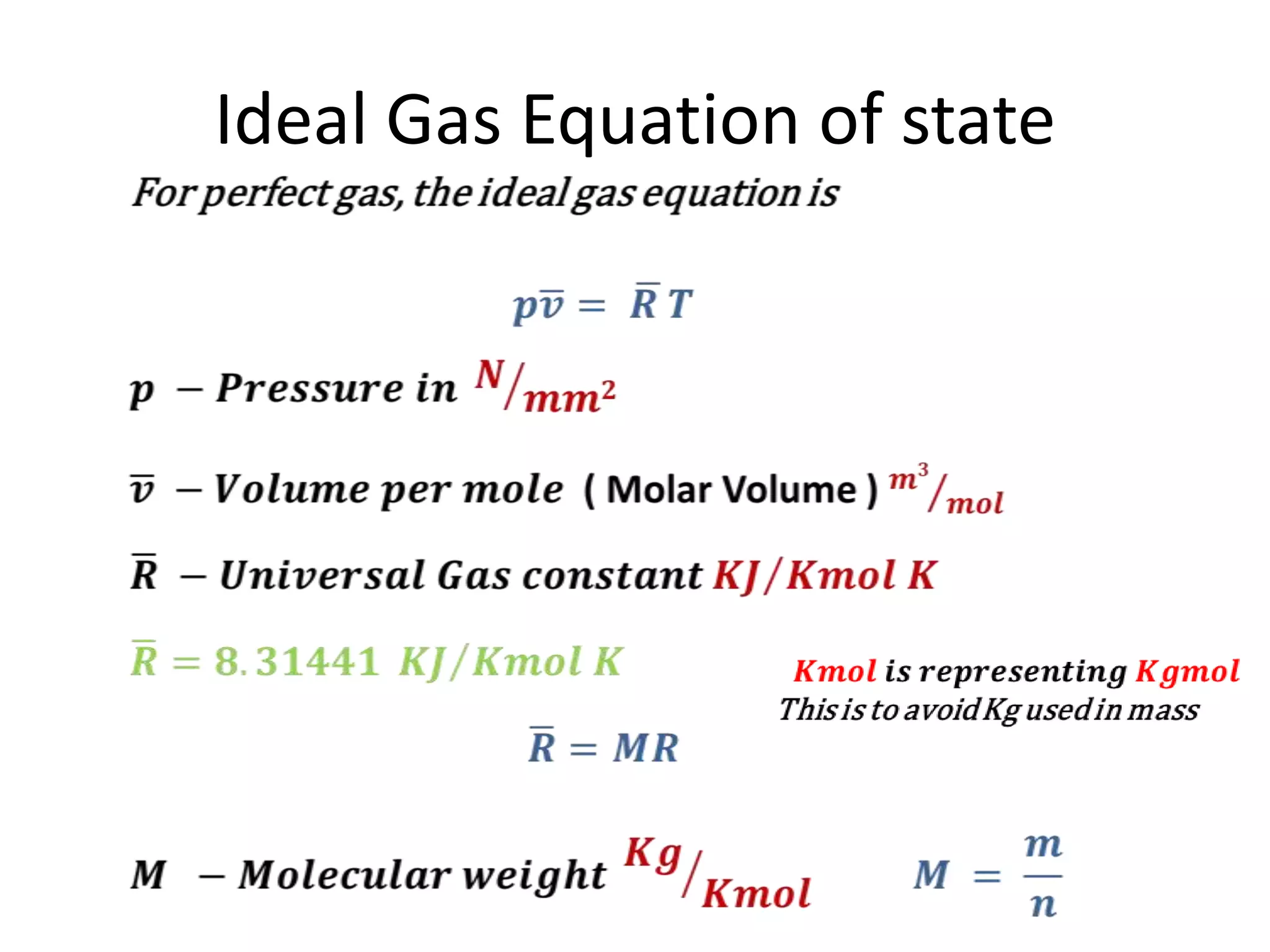
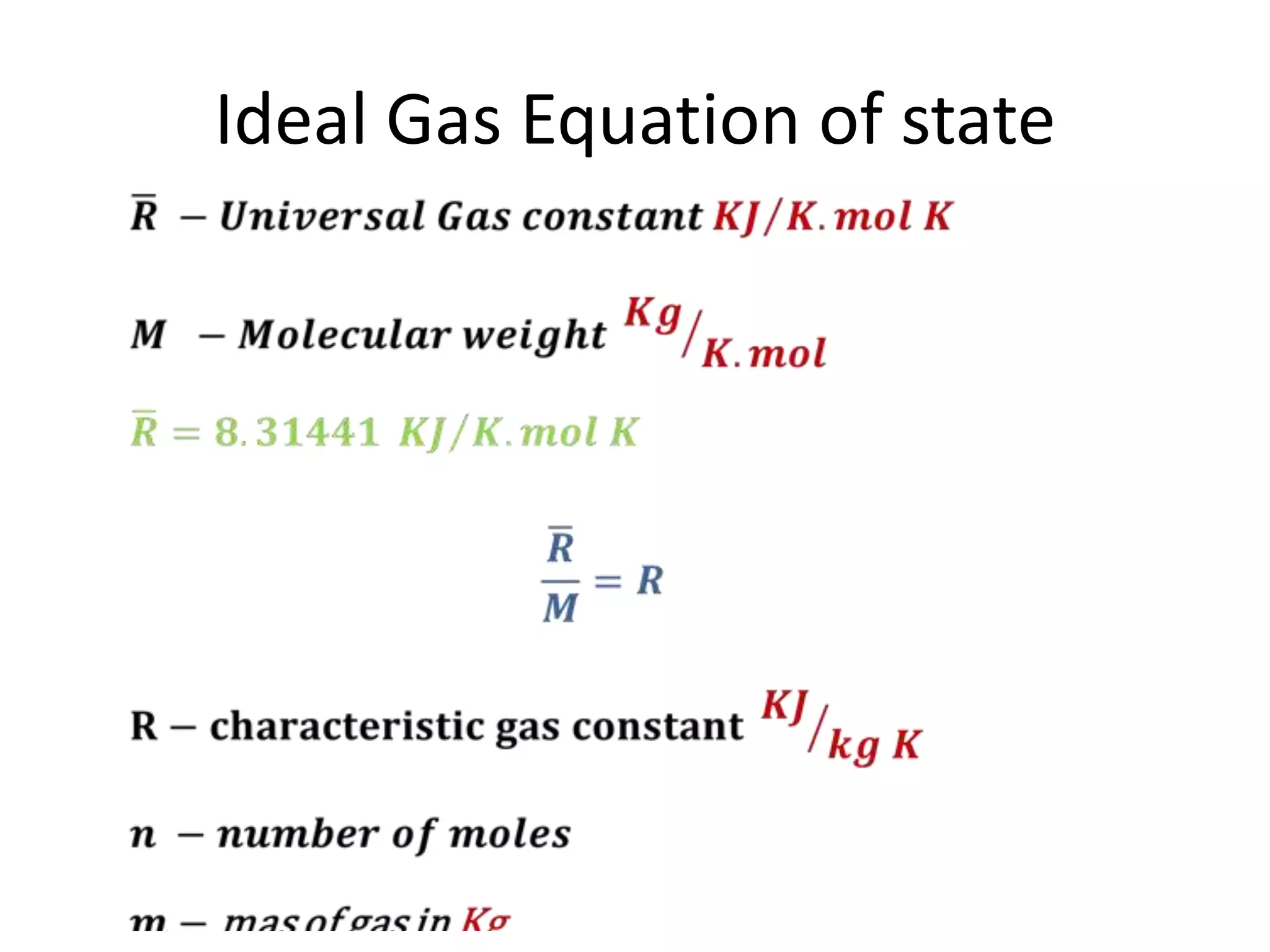
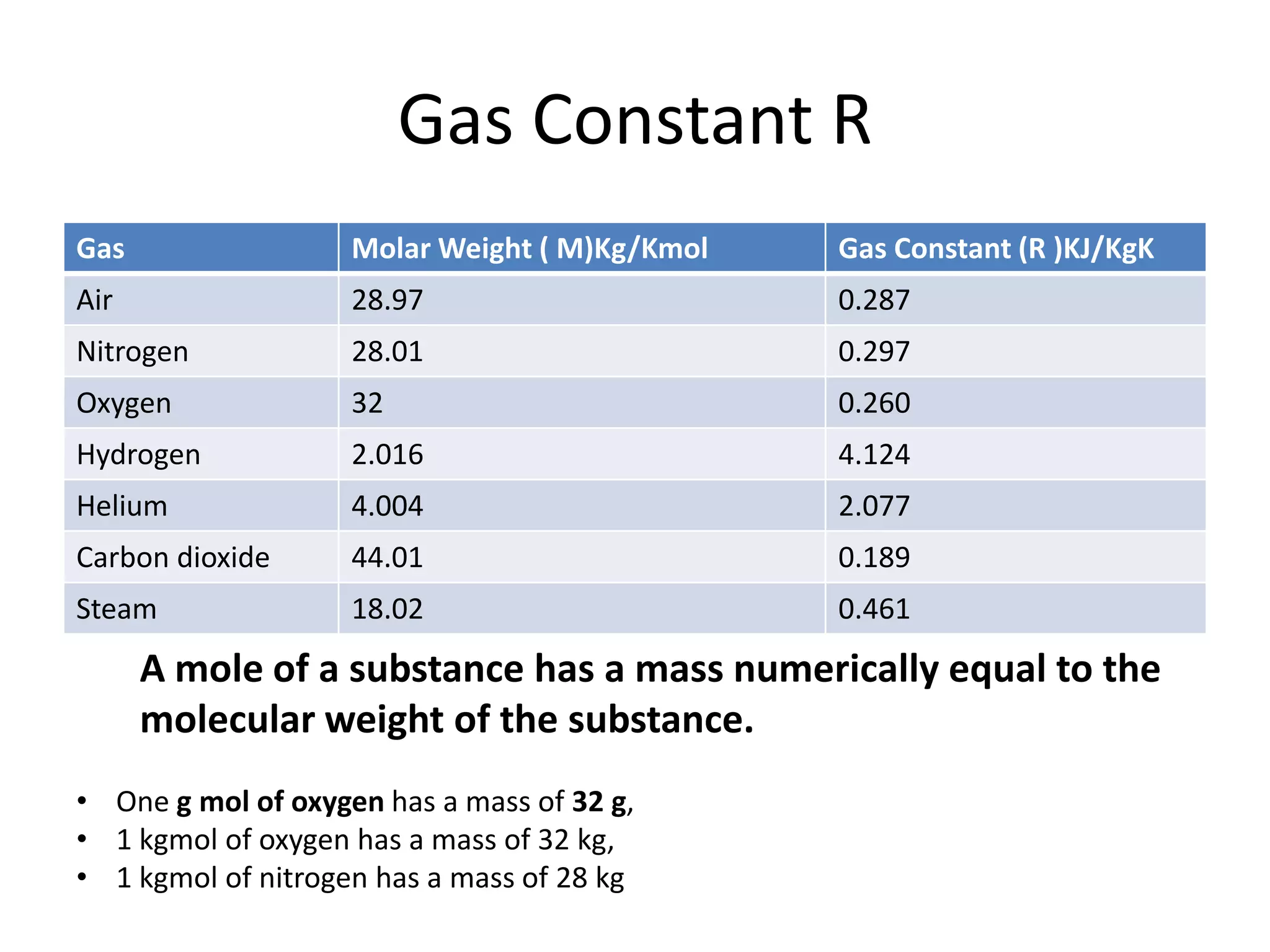
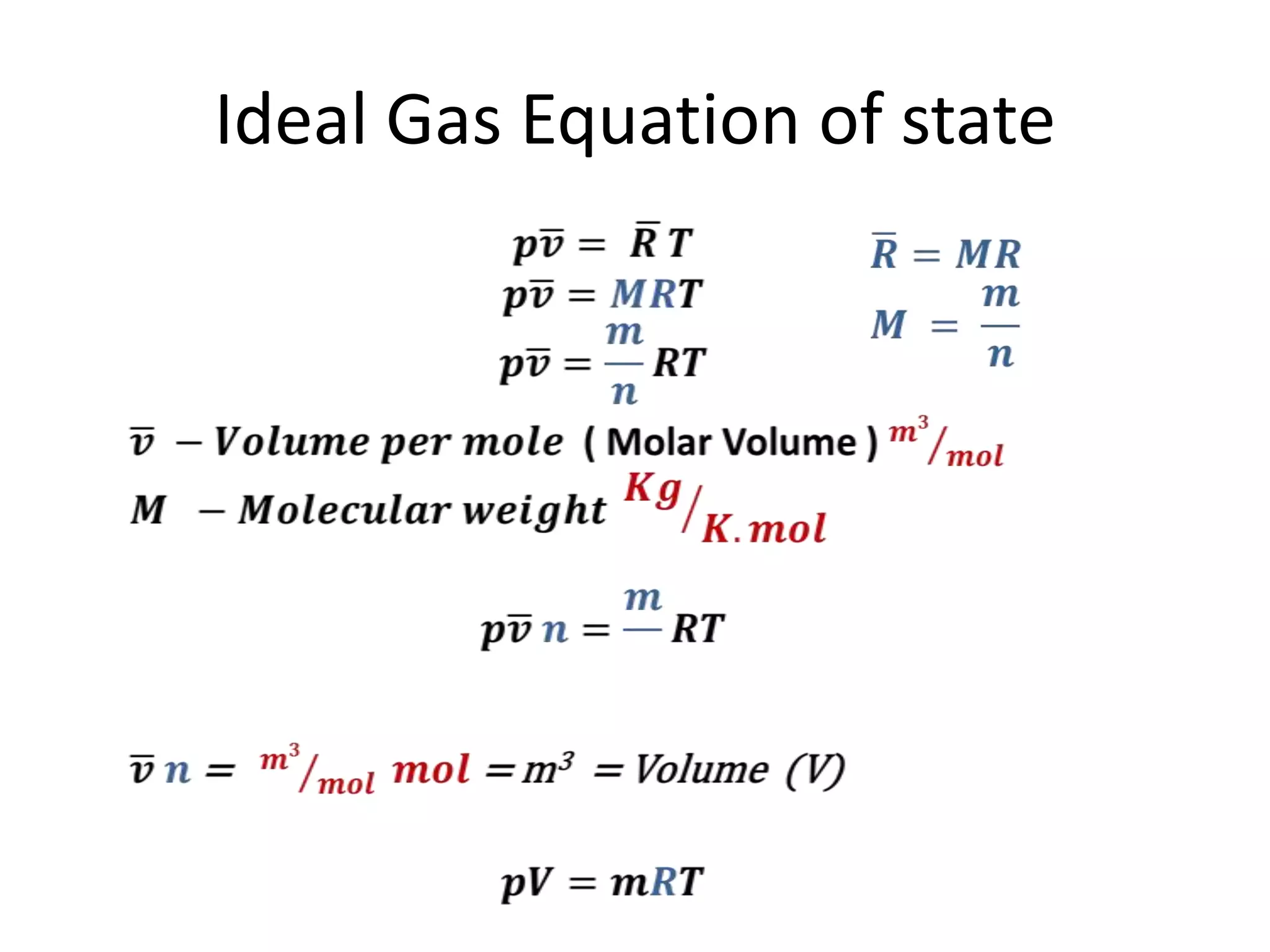
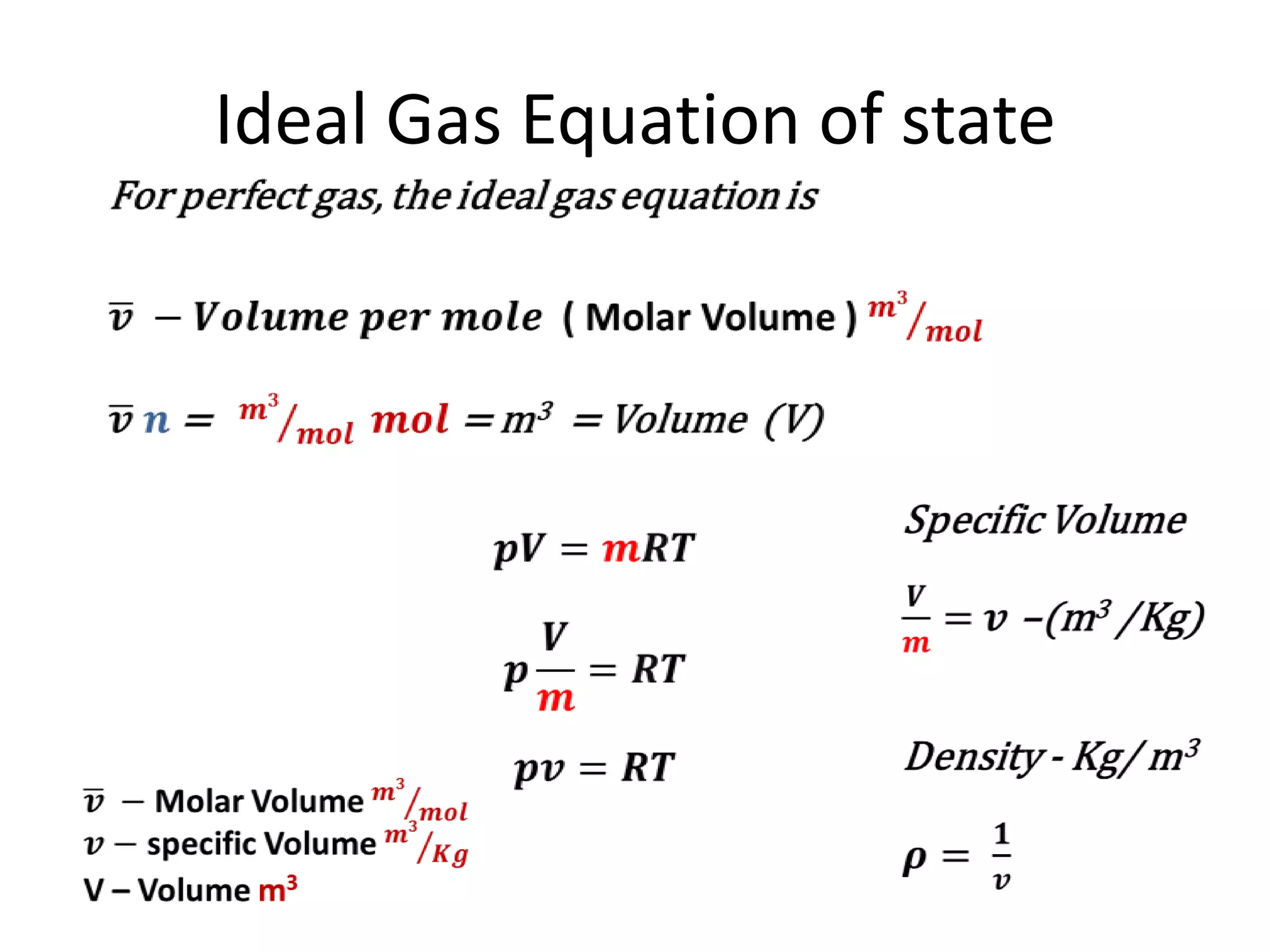
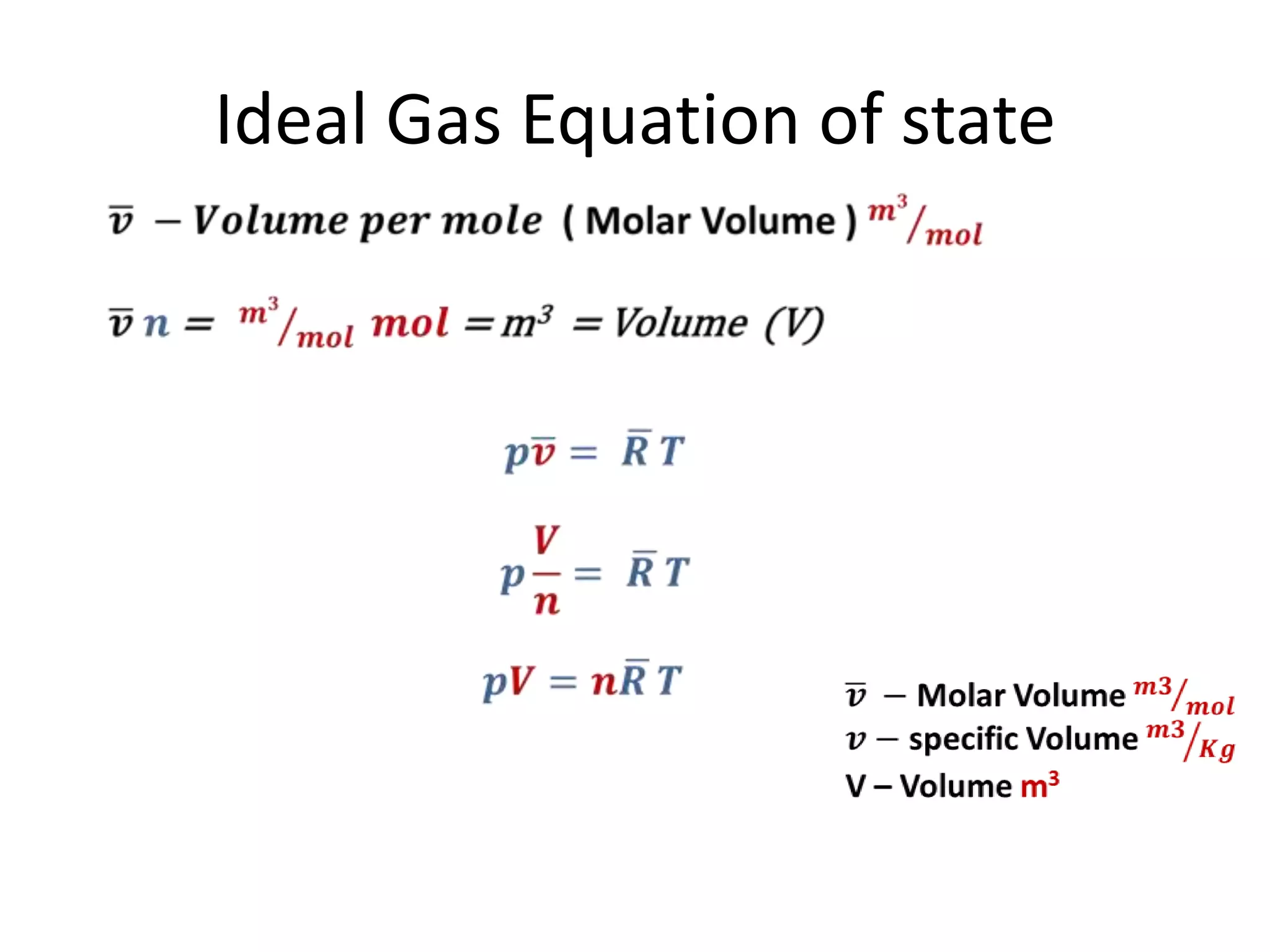
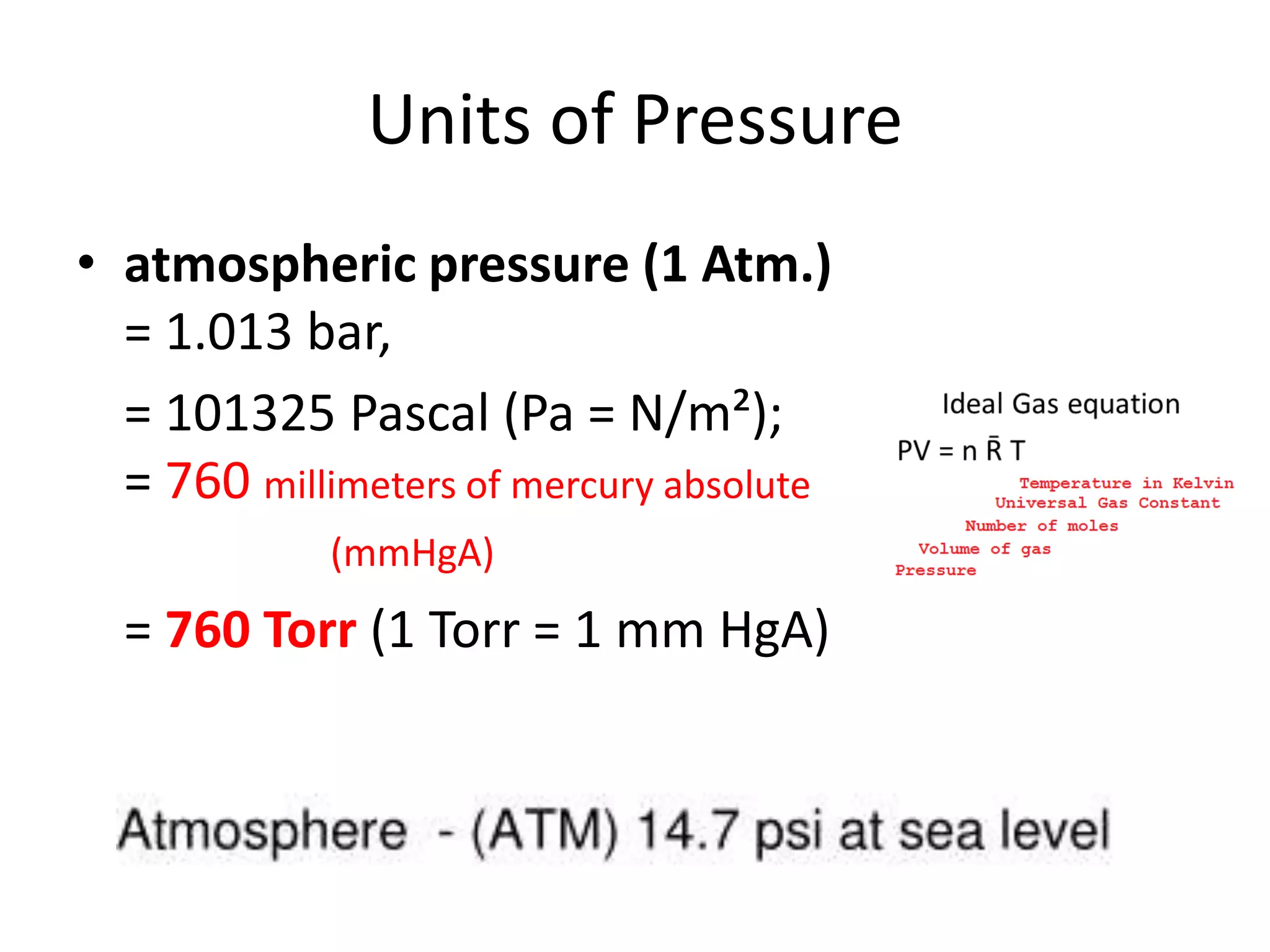
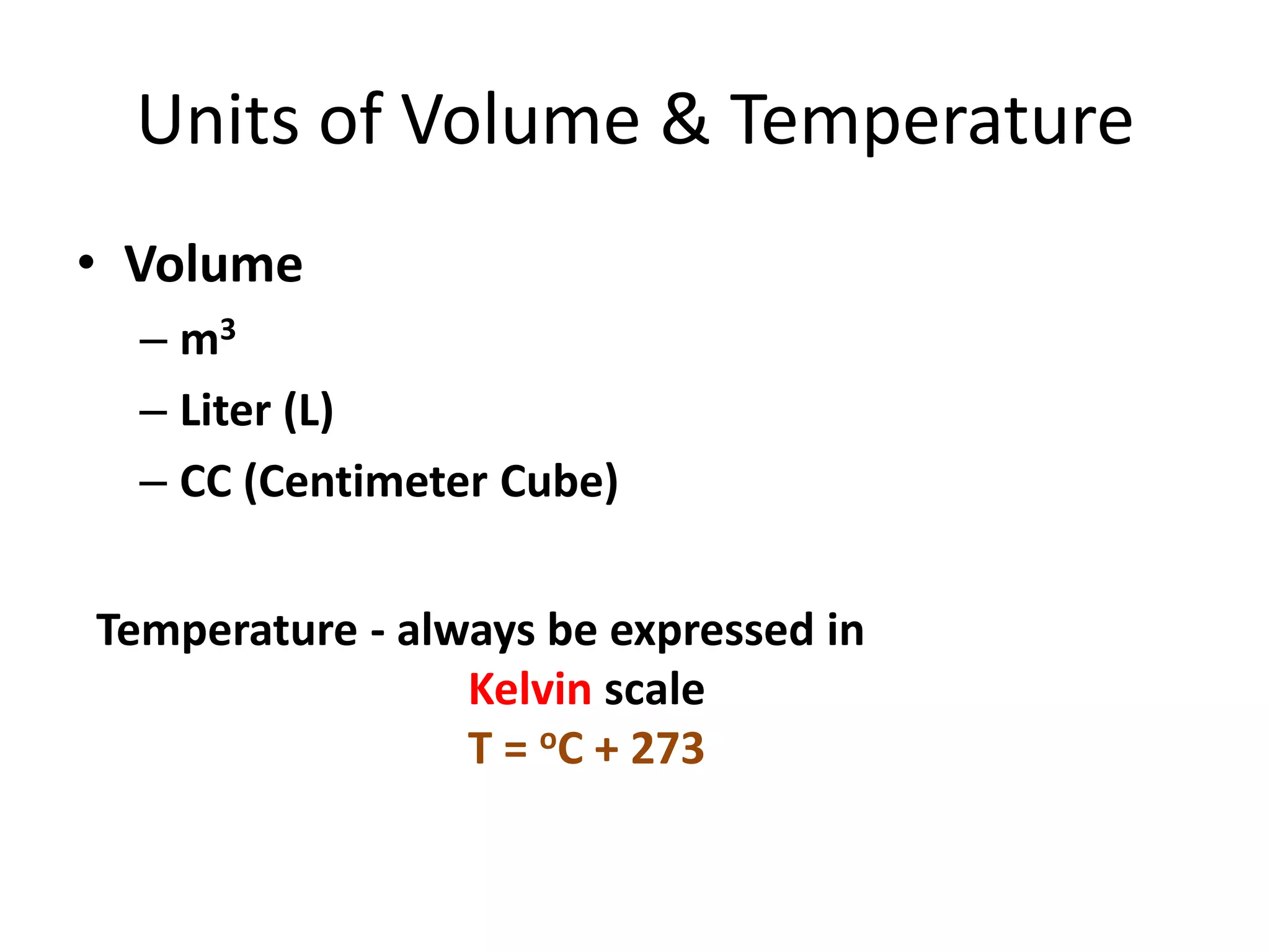
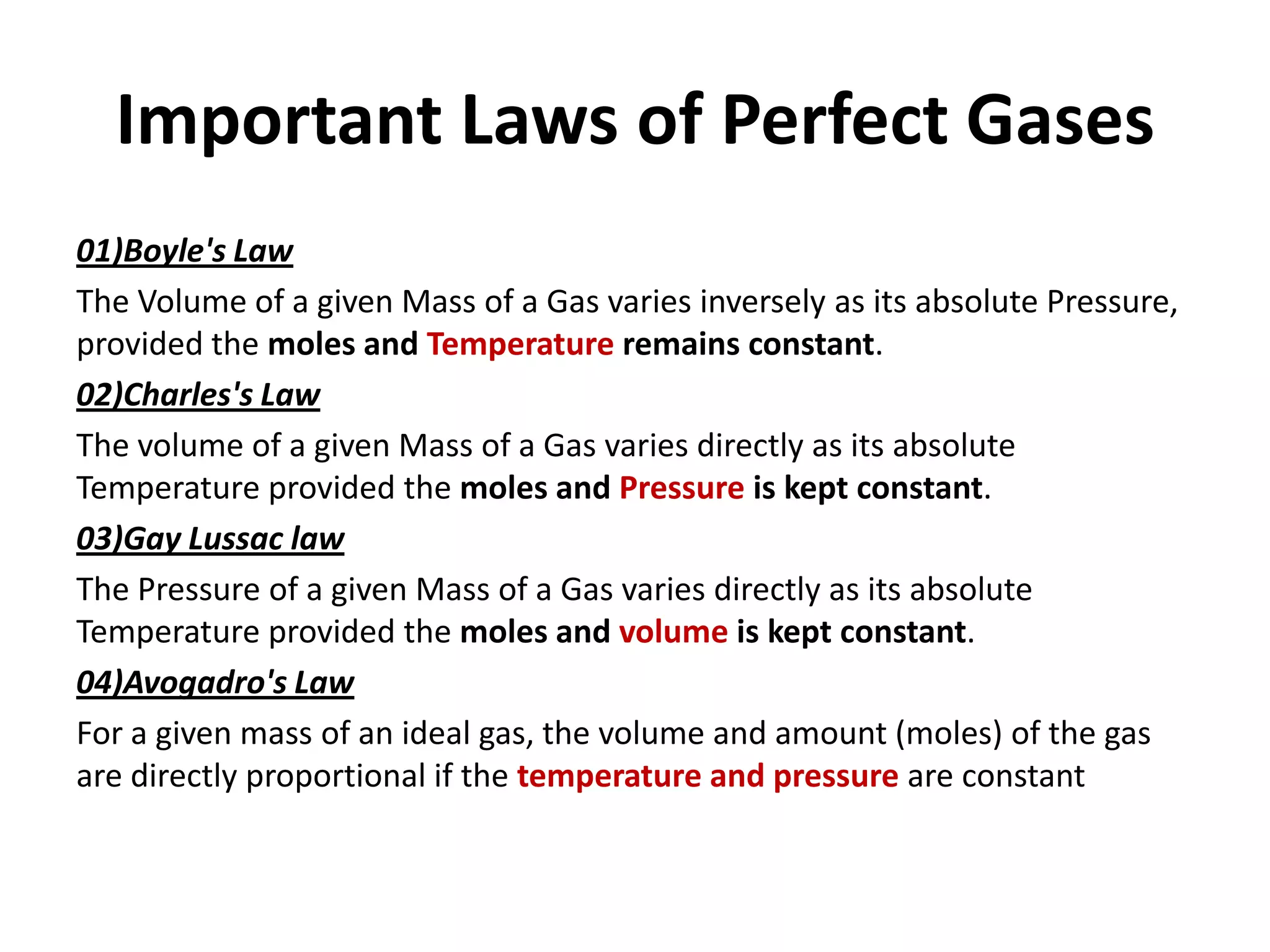
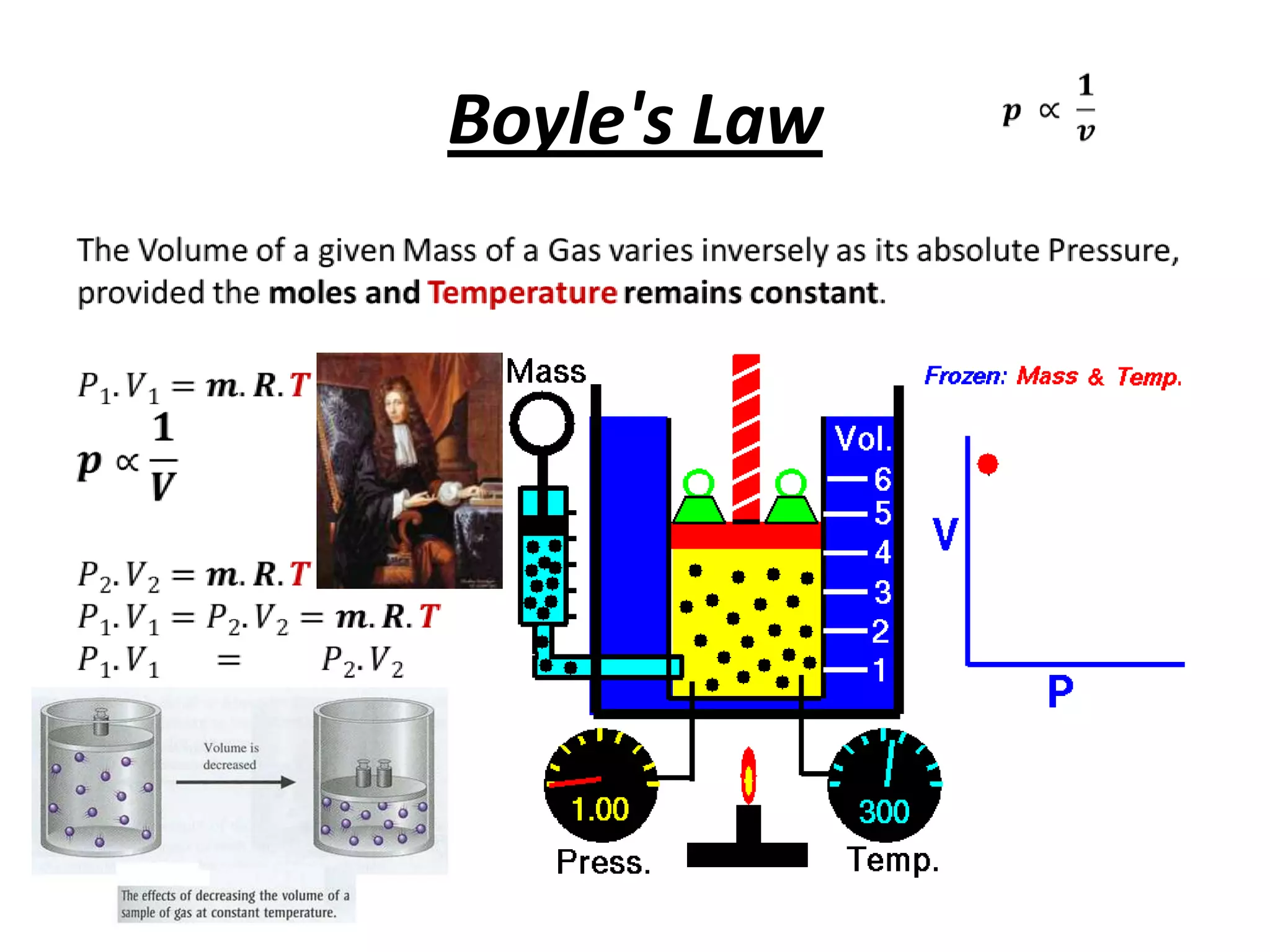
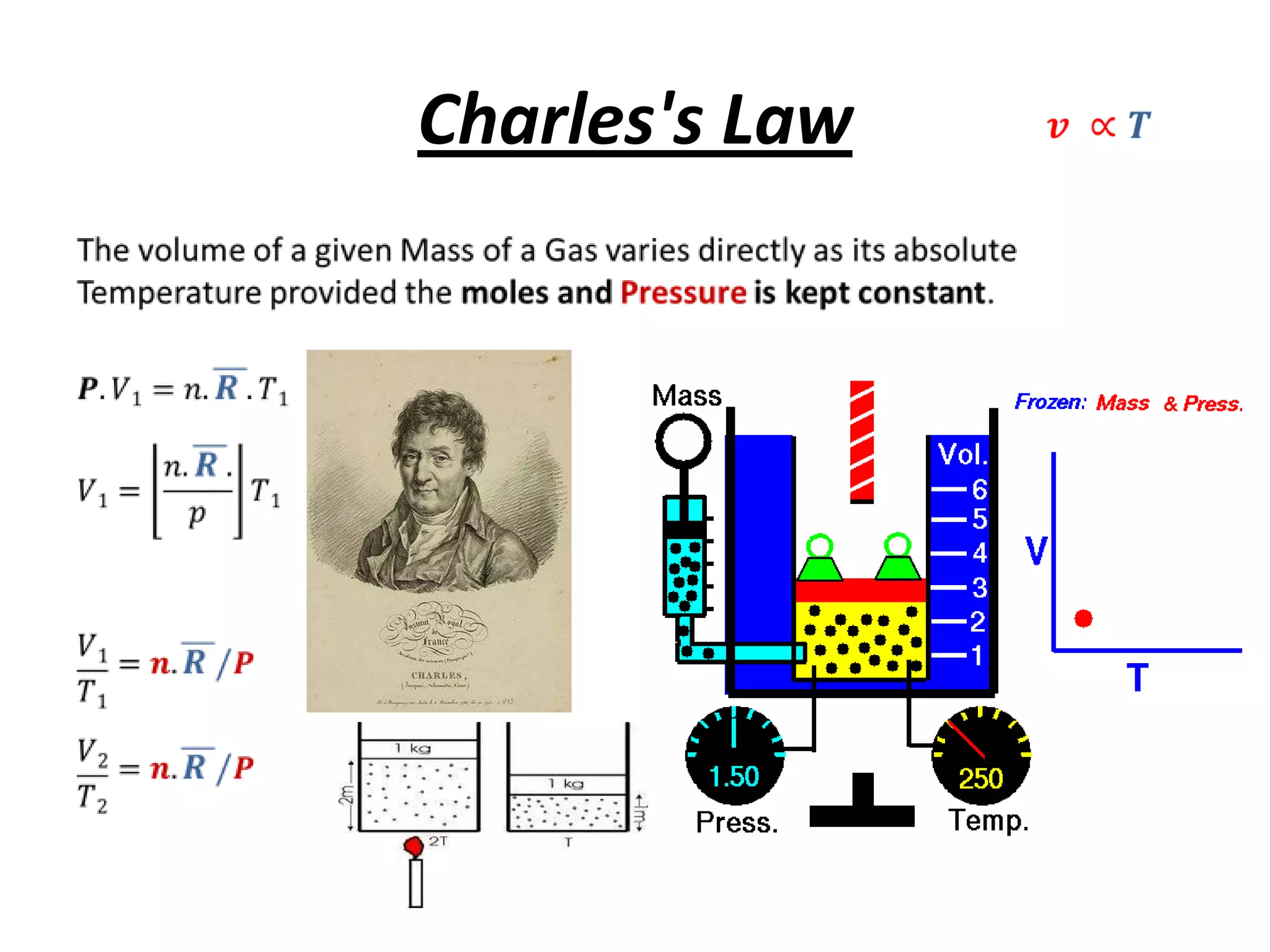
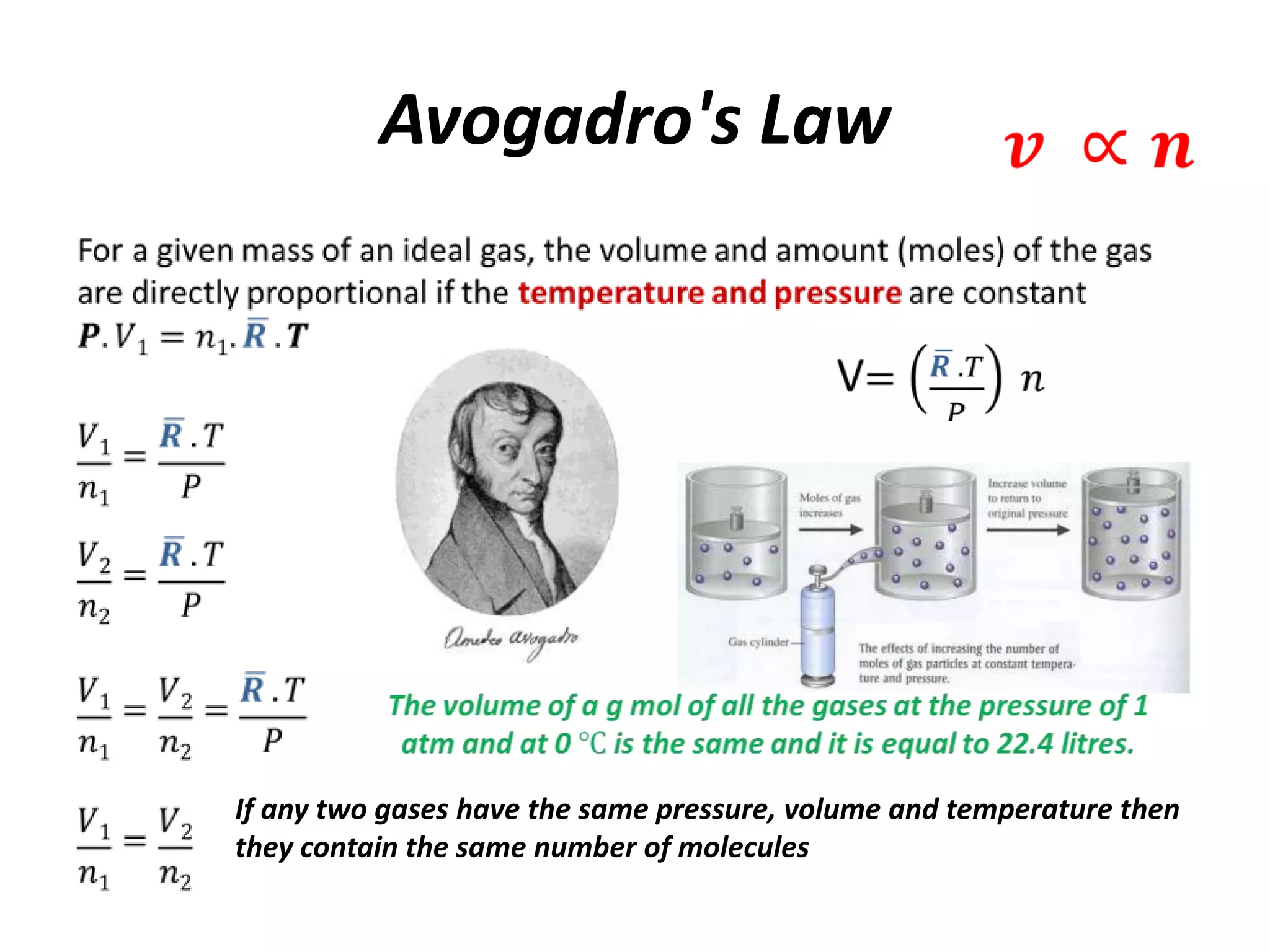
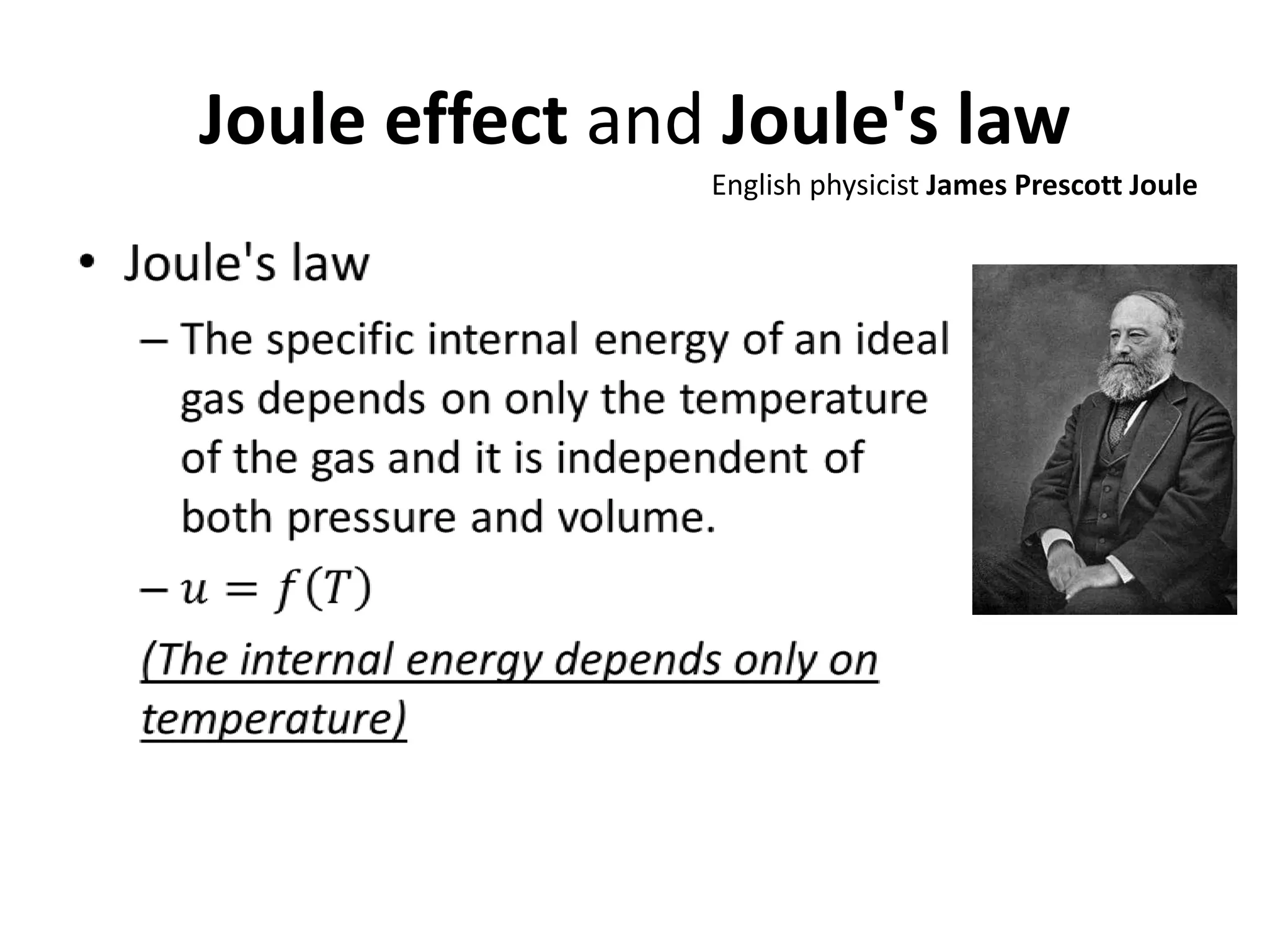
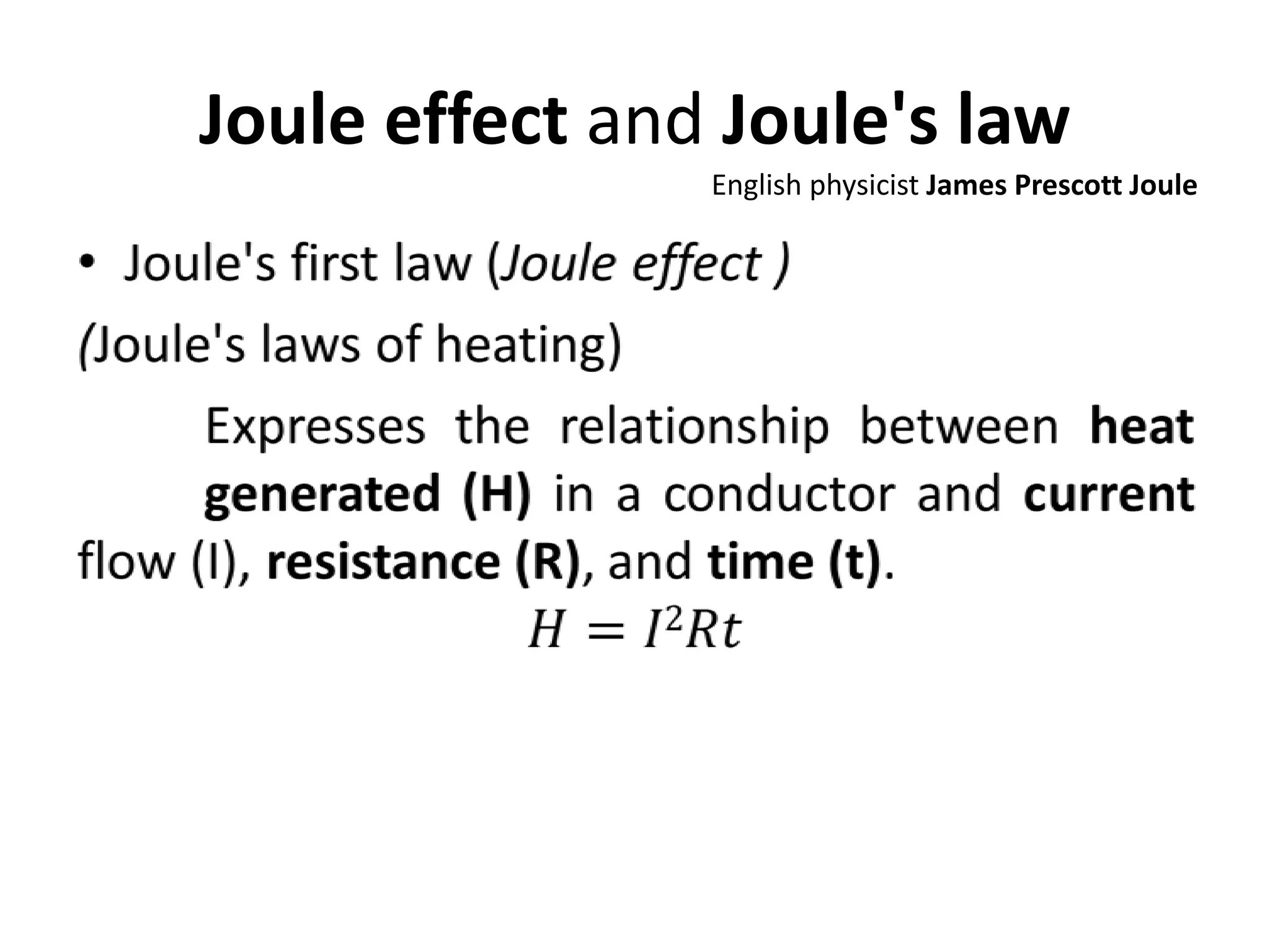
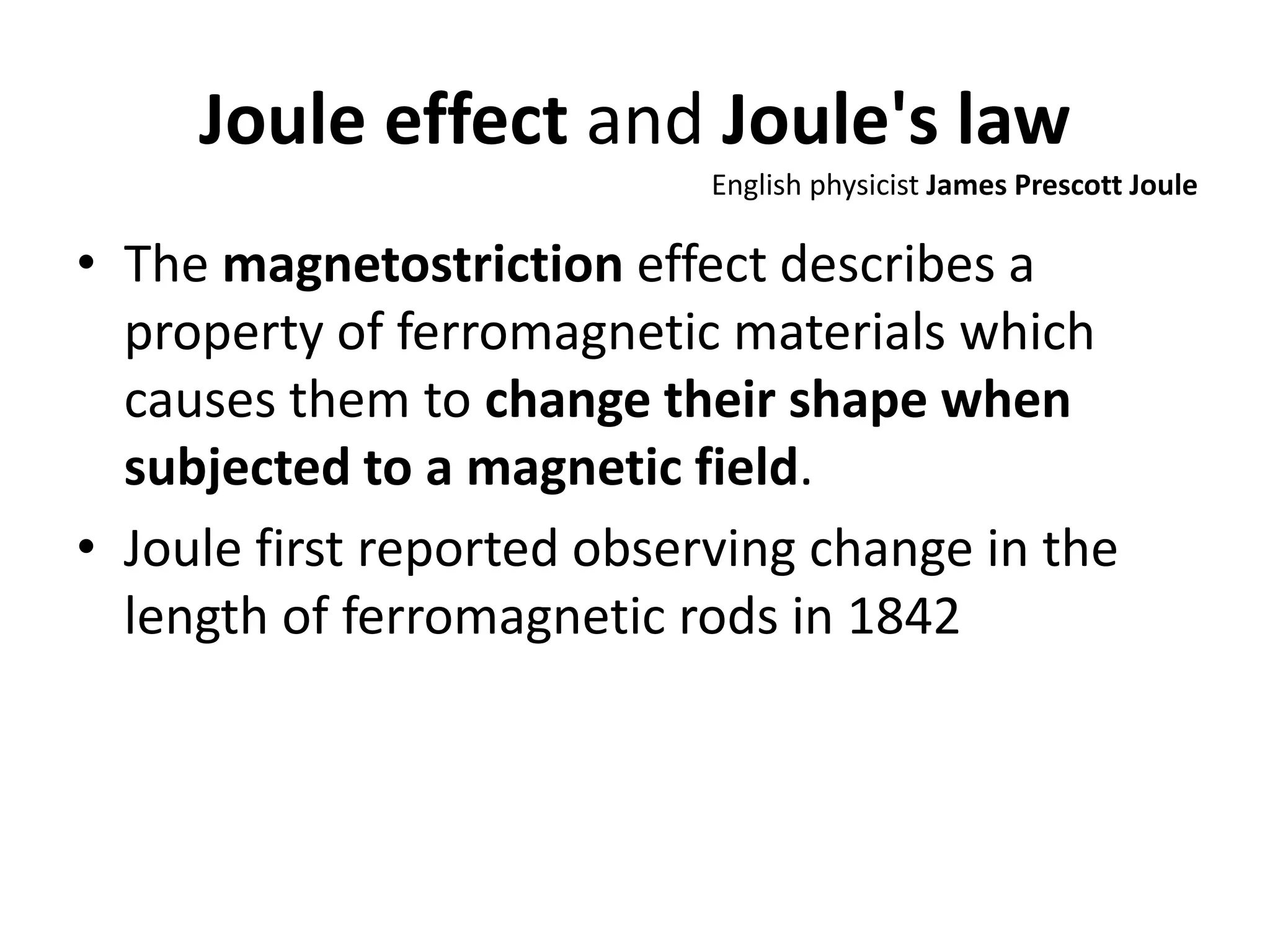
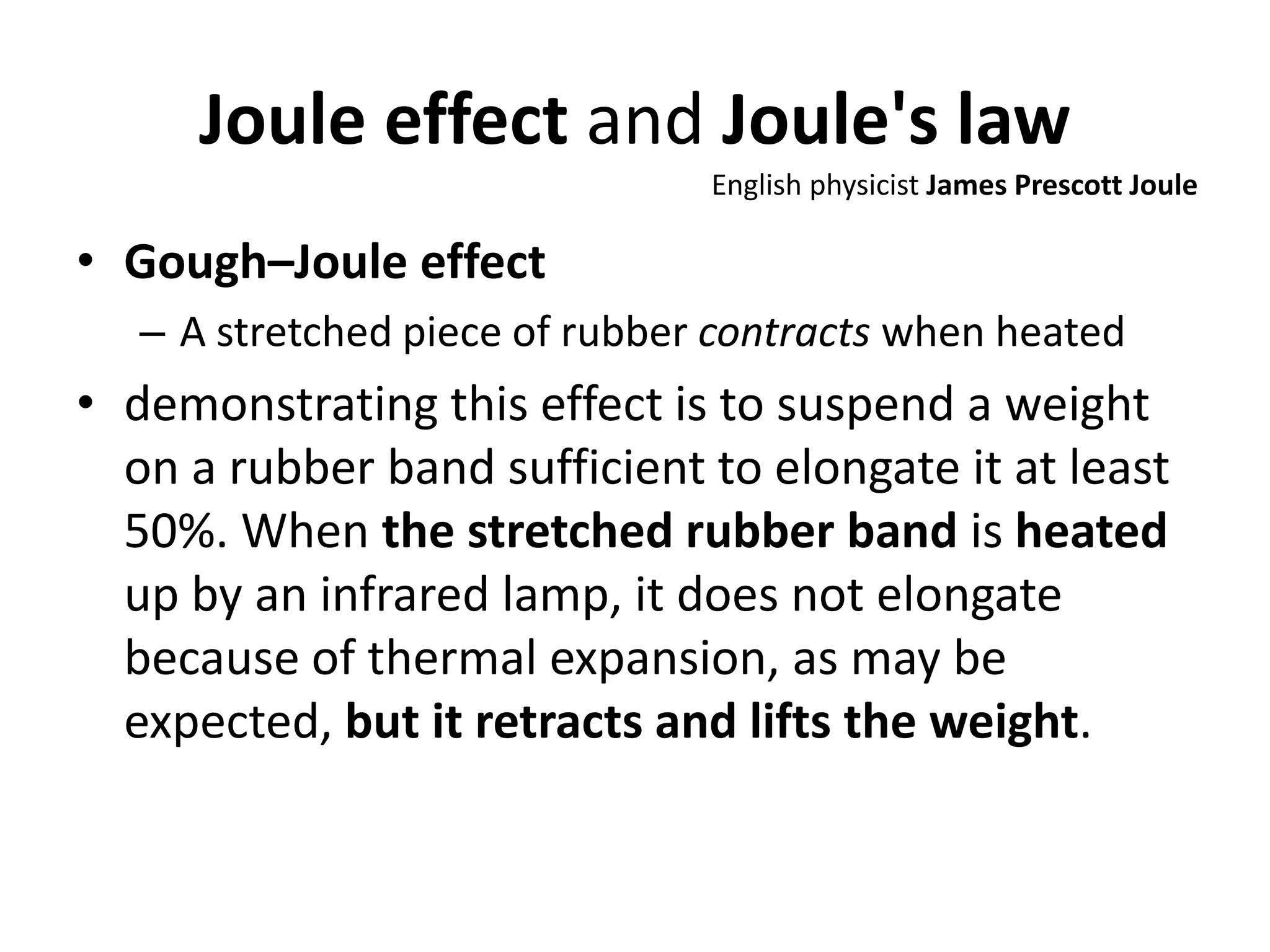
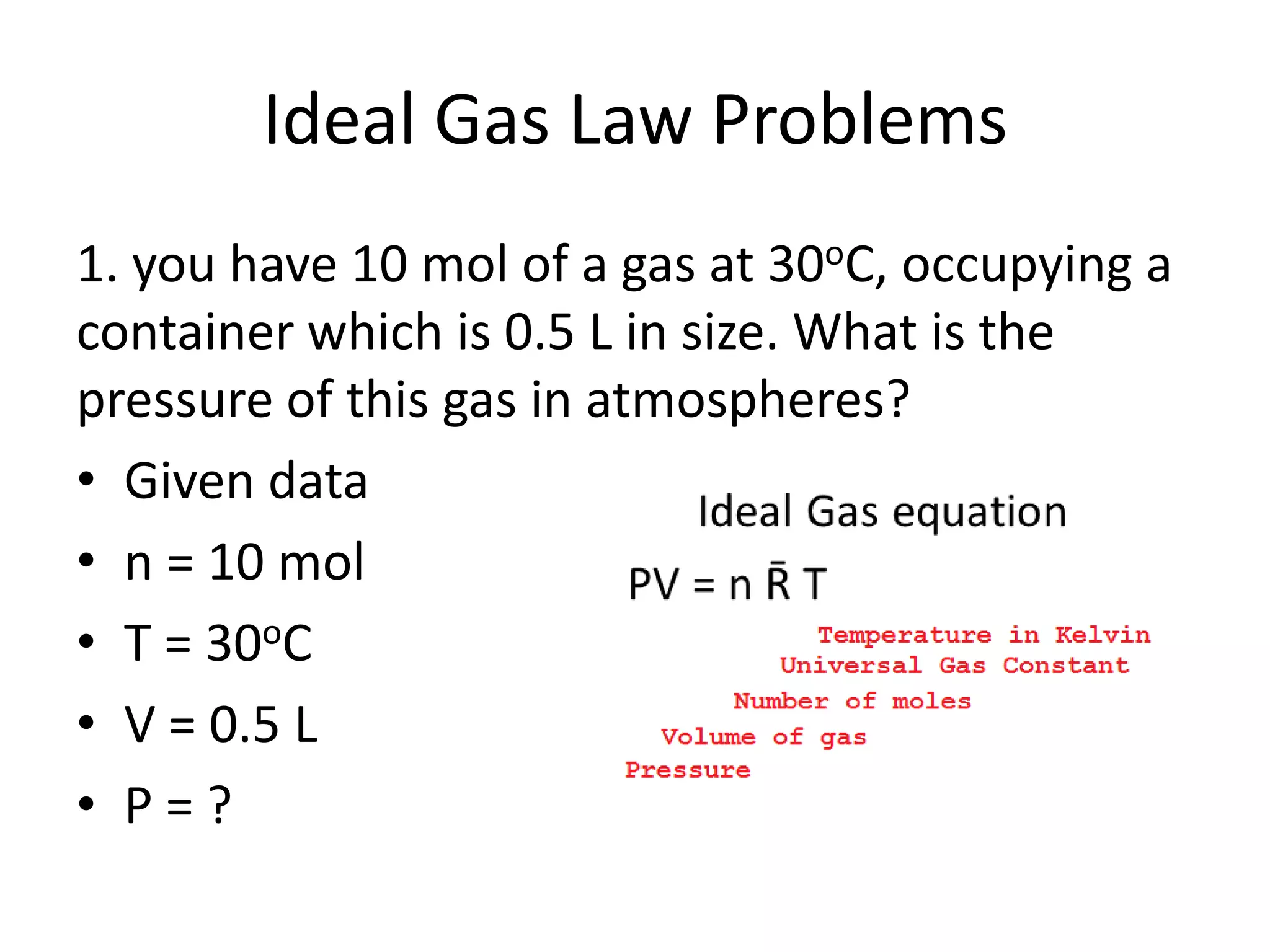
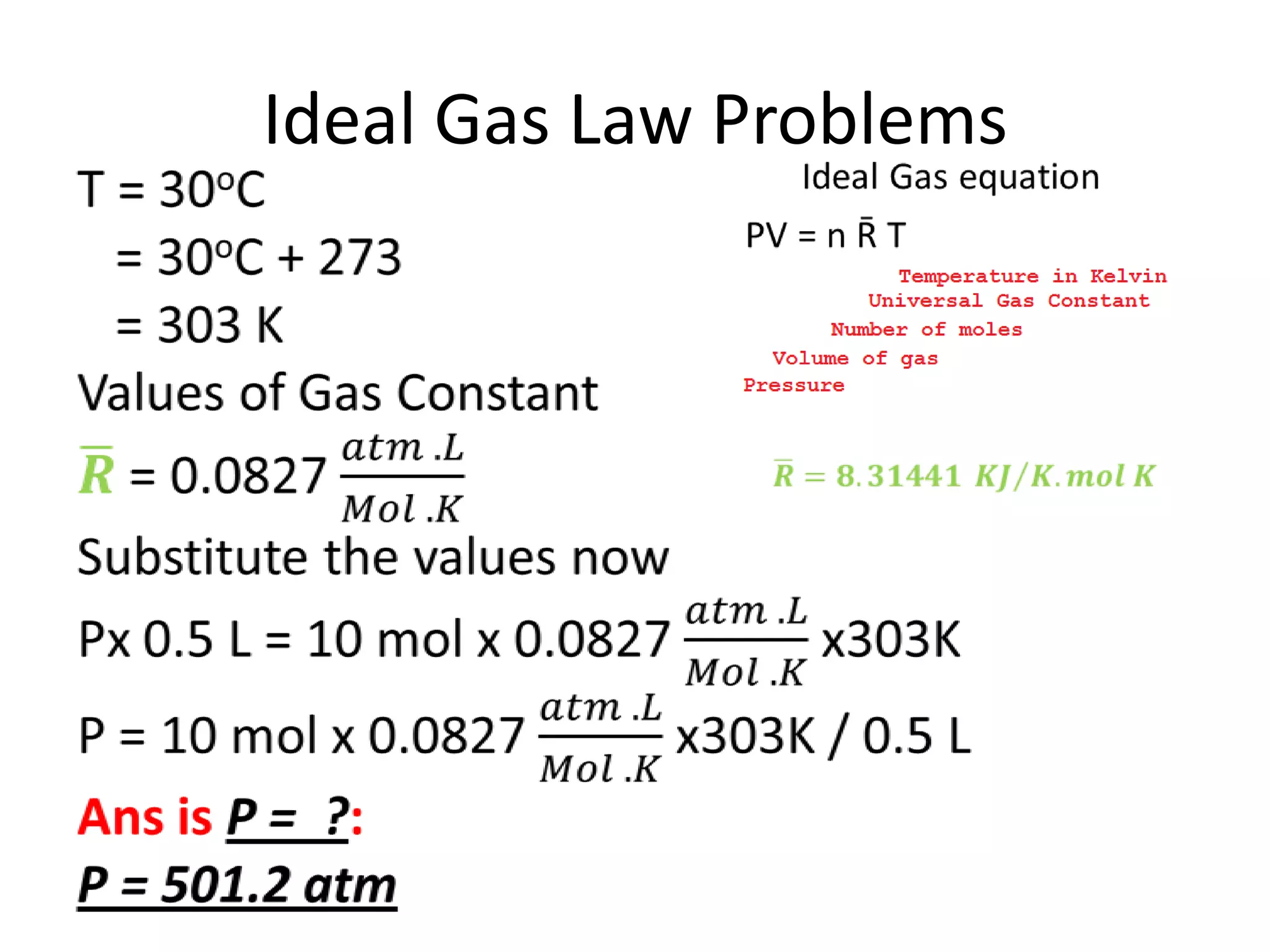
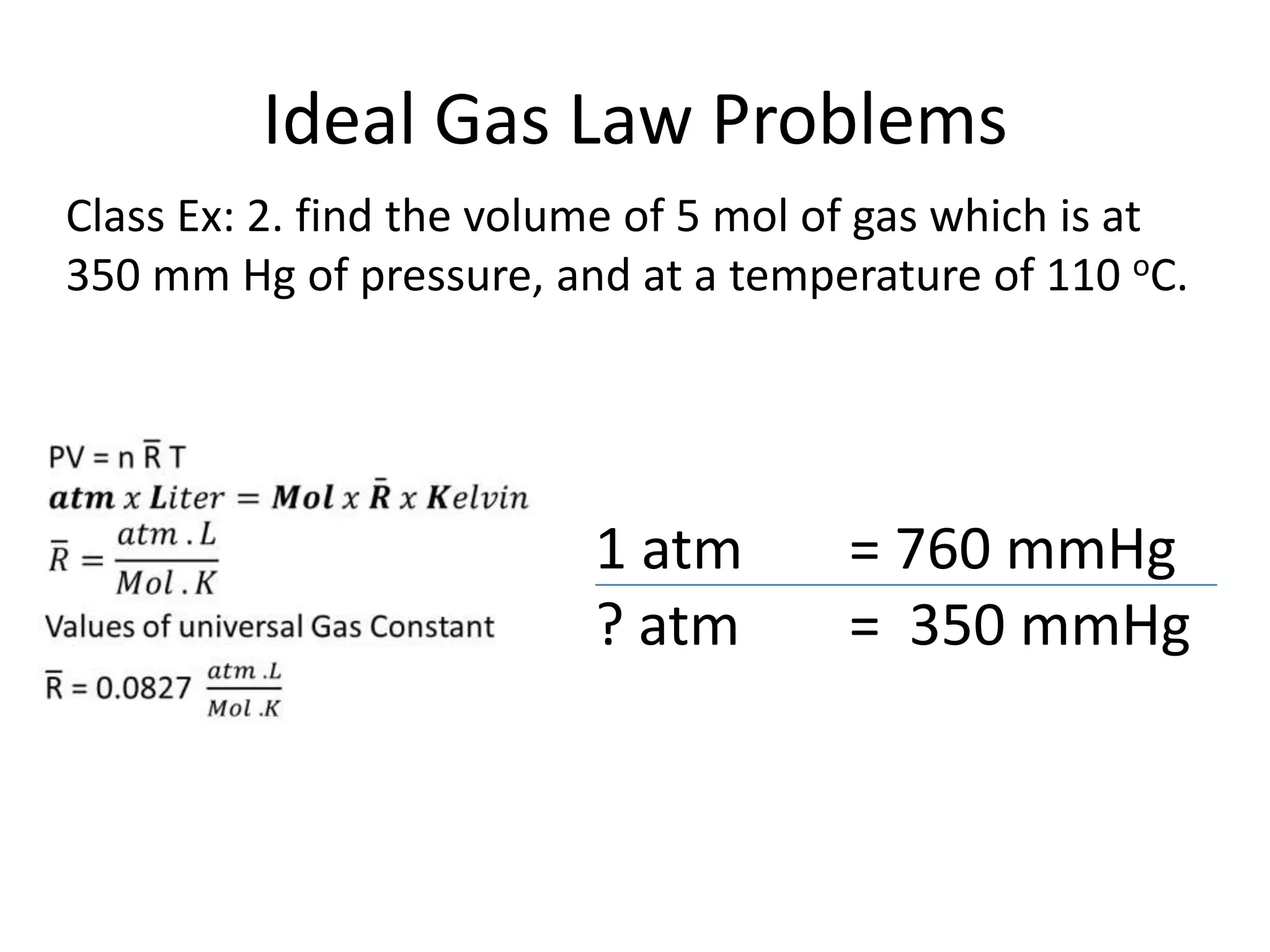
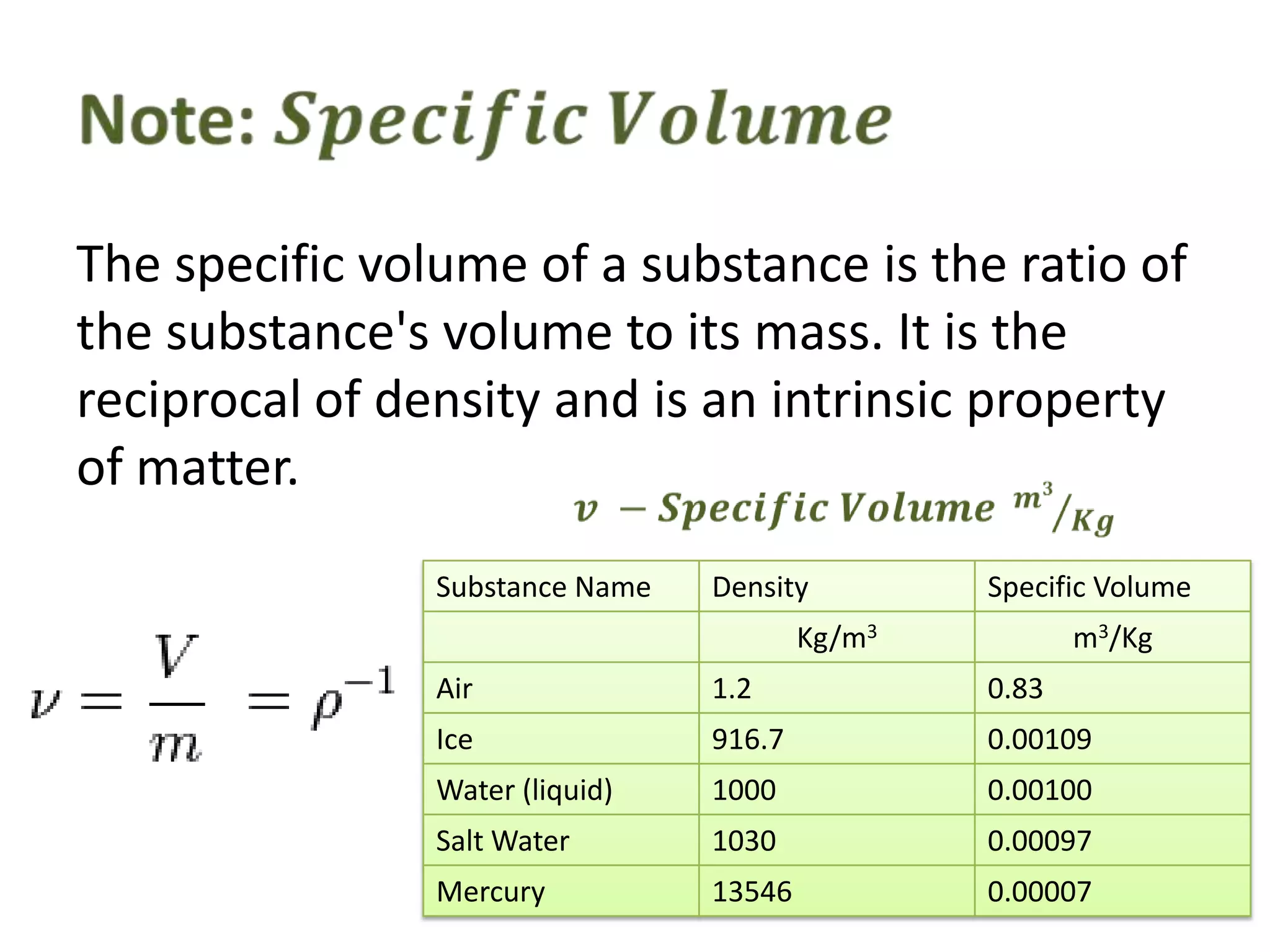
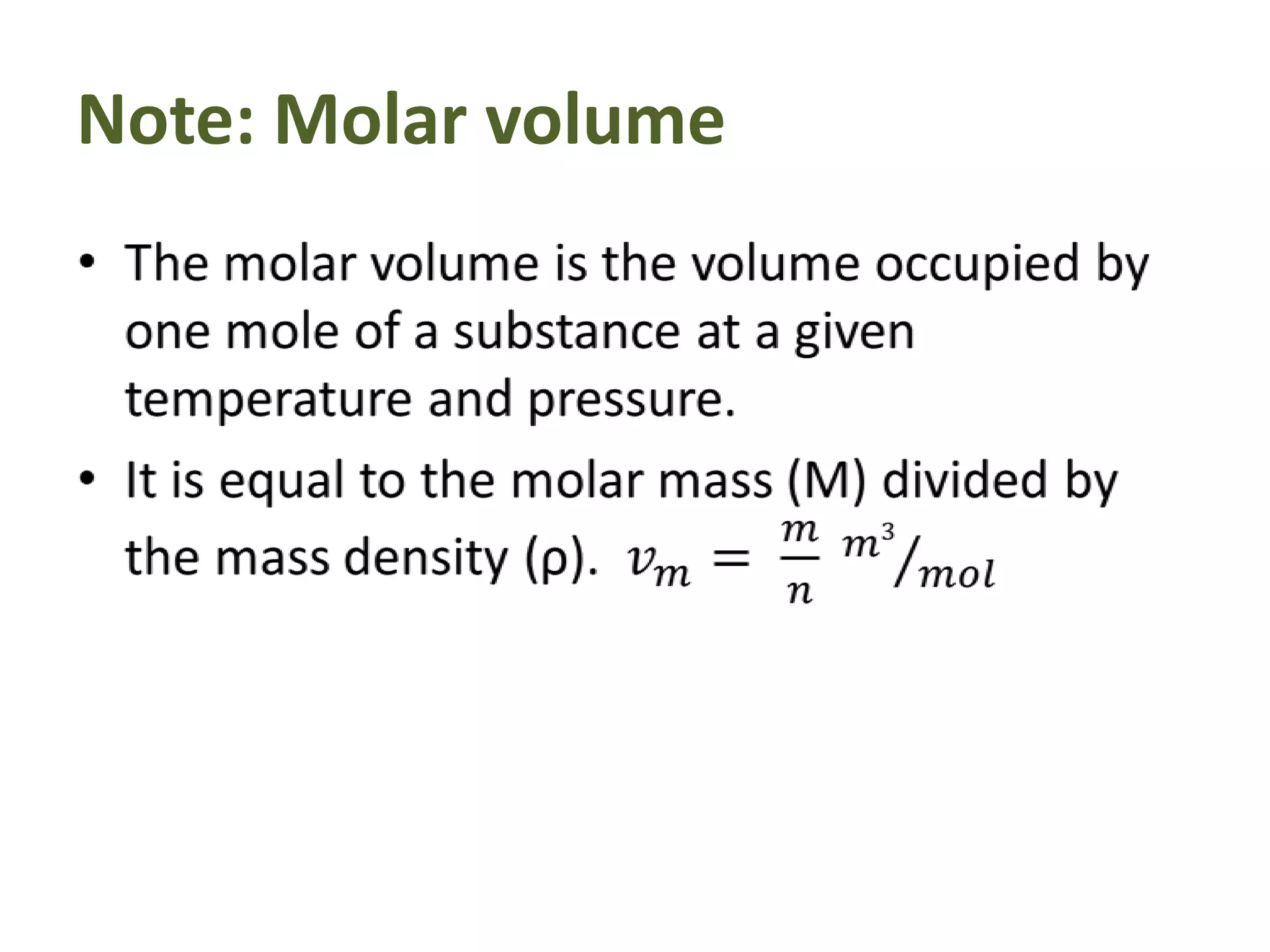
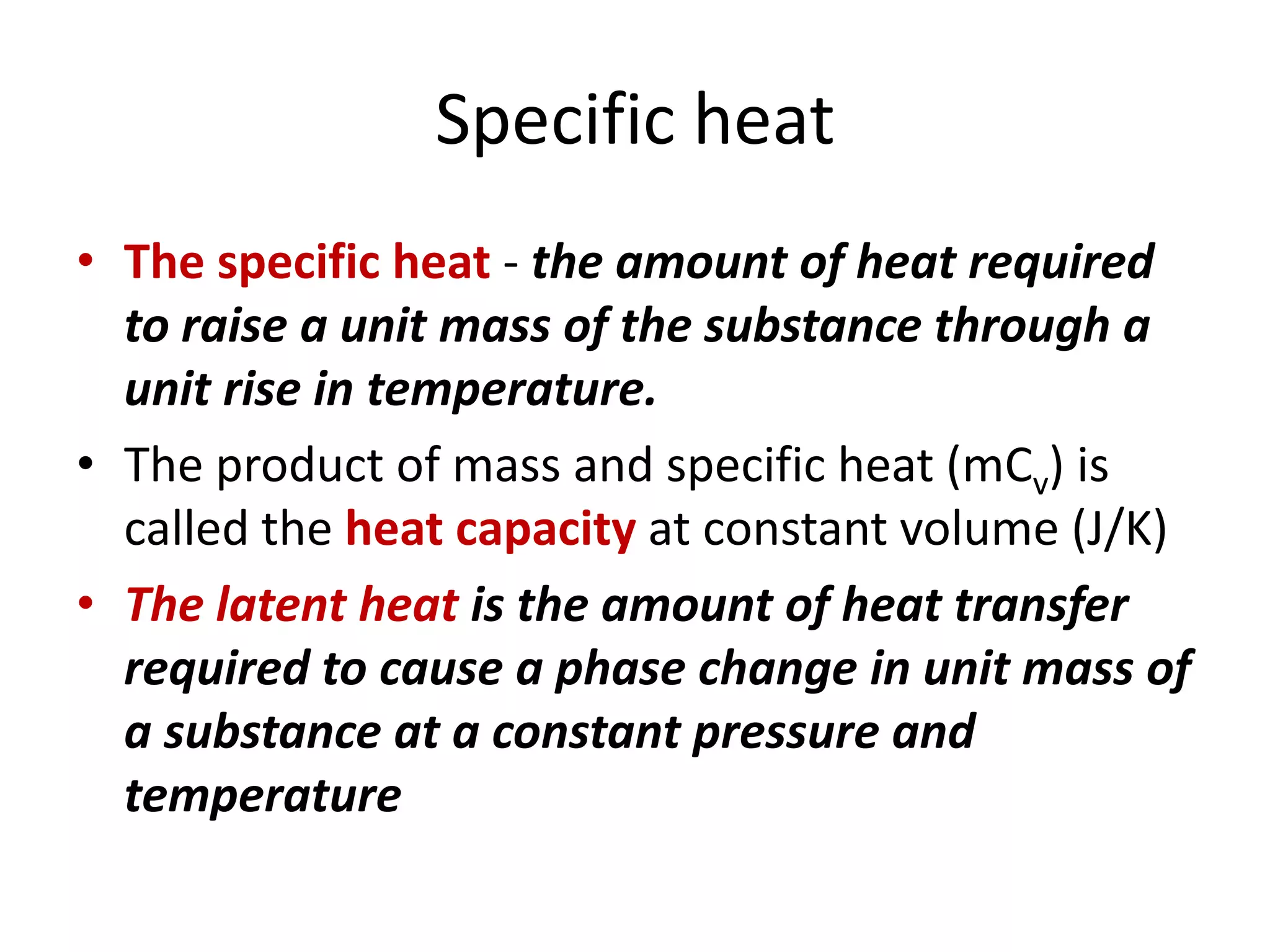
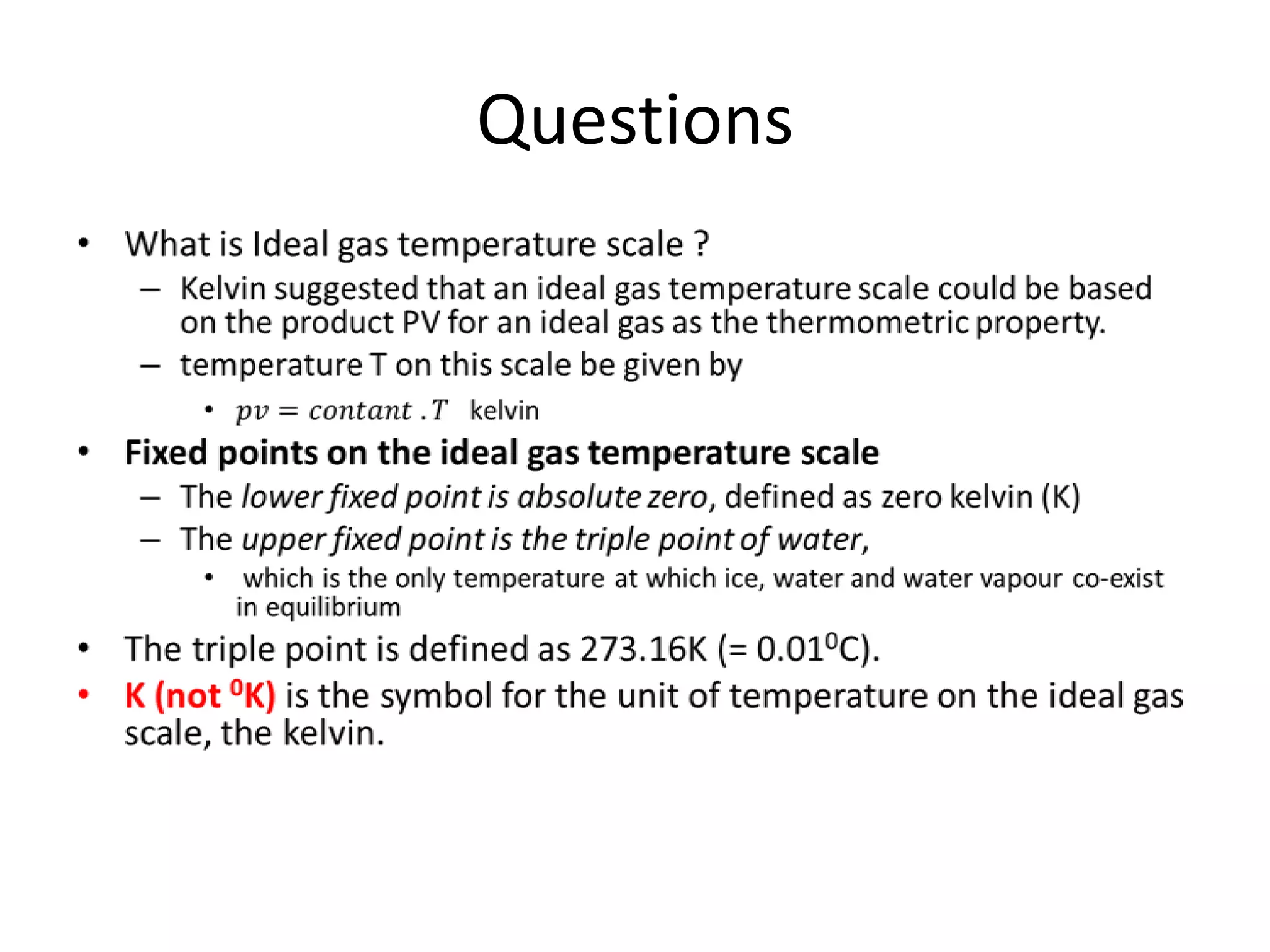
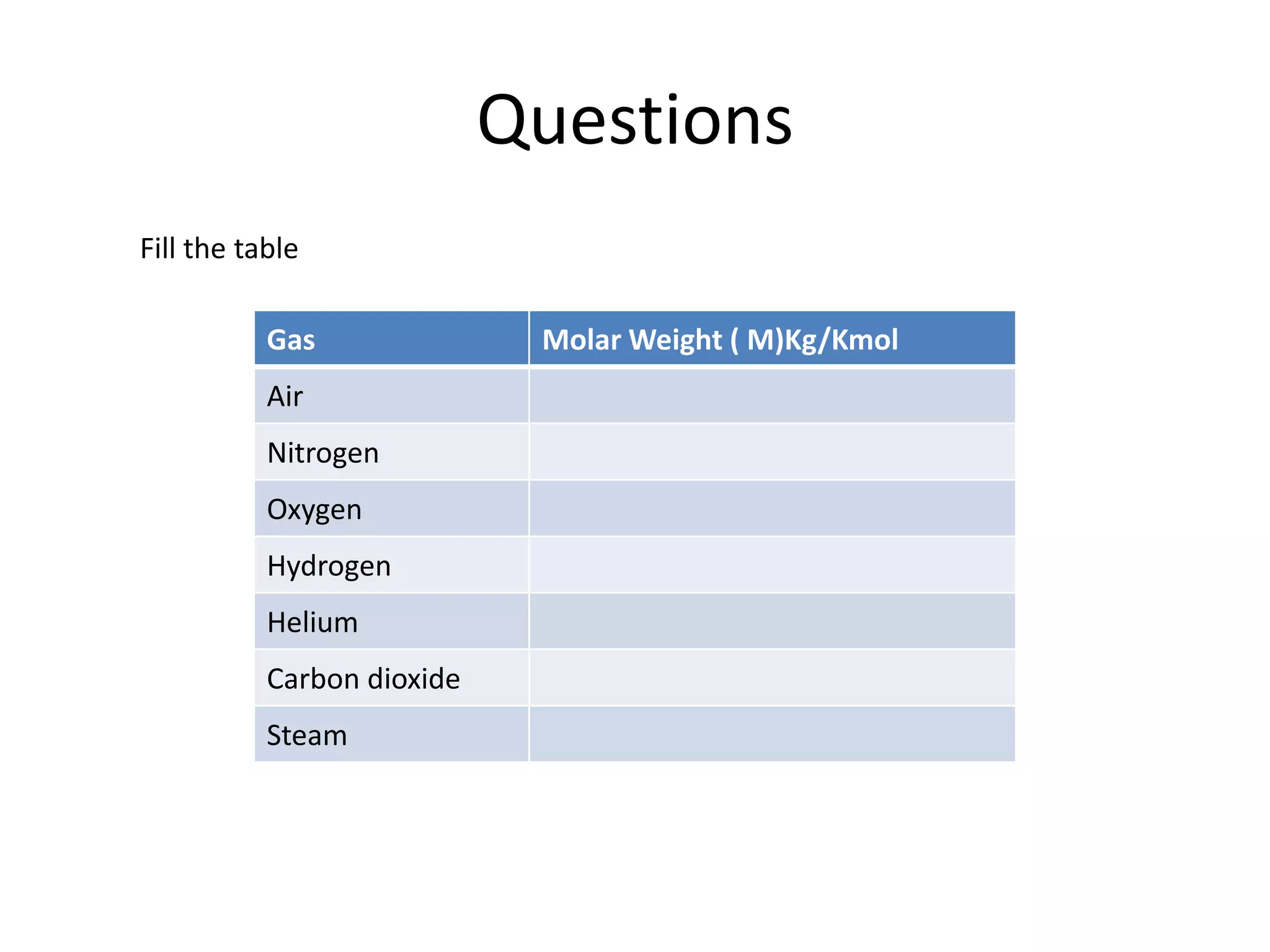
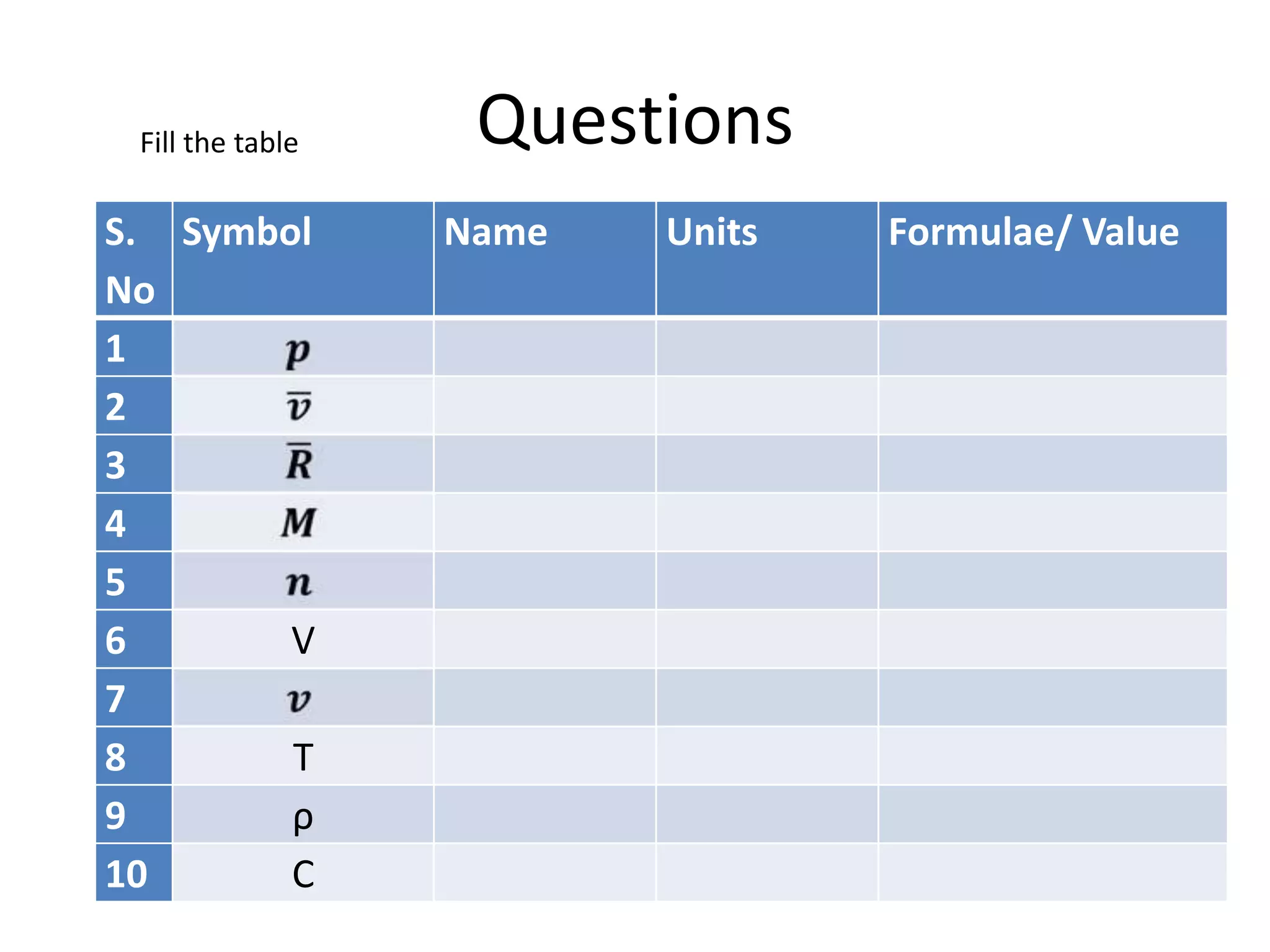
This document discusses ideal gases and their properties. It defines an ideal gas as a theoretical gas composed of point-like particles that obey gas laws perfectly. Some key properties of ideal gases are that they have negligible volume and mass and undergo perfectly elastic collisions. The document also covers gas laws such as Boyle's, Charles', and Gay-Lussac's laws. It provides the ideal gas equation of state and defines terms like molar mass, gas constant, pressure units and heat capacities. Several example problems applying the ideal gas law are also included.