Embed presentation
Downloaded 68 times


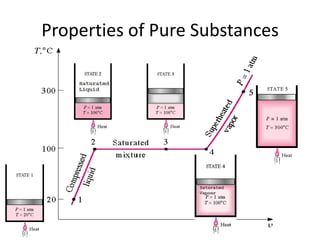
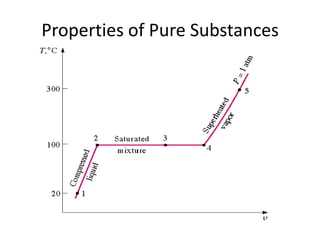
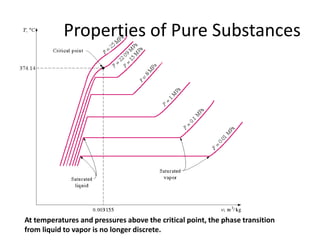
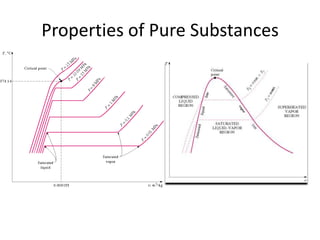
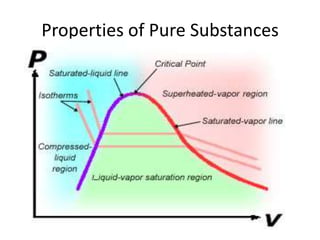
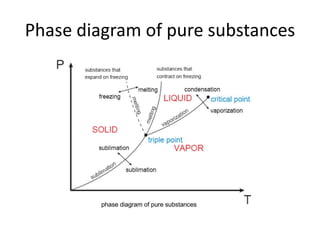


A pure substance has a homogeneous and invariable chemical composition and may exist in more than one phase, such as water existing in solid, liquid, and vapor phases. At temperatures and pressures above the critical point, the discrete phase transition from liquid to vapor is no longer present. Phase diagrams can be used to represent the different phases of pure substances at various temperatures and pressures.









