Embed presentation
Downloaded 31 times

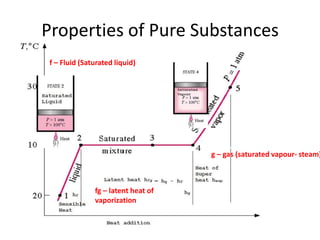


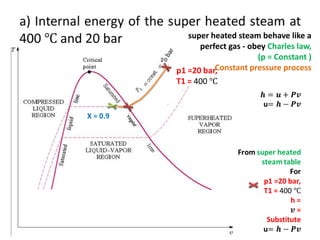





This document discusses properties of pure substances and provides three homework problems. It defines latent heat of vaporization and discusses how superheated steam behaves like an ideal gas obeying Charles' Law. The first problem asks to calculate the internal energy of wet steam at a dryness fraction of 0.9 under constant pressure. The second problem asks to explain Van der Waals' equation, Clausius equation, and Dieterici equation. References for further reading on thermodynamics are also provided.








