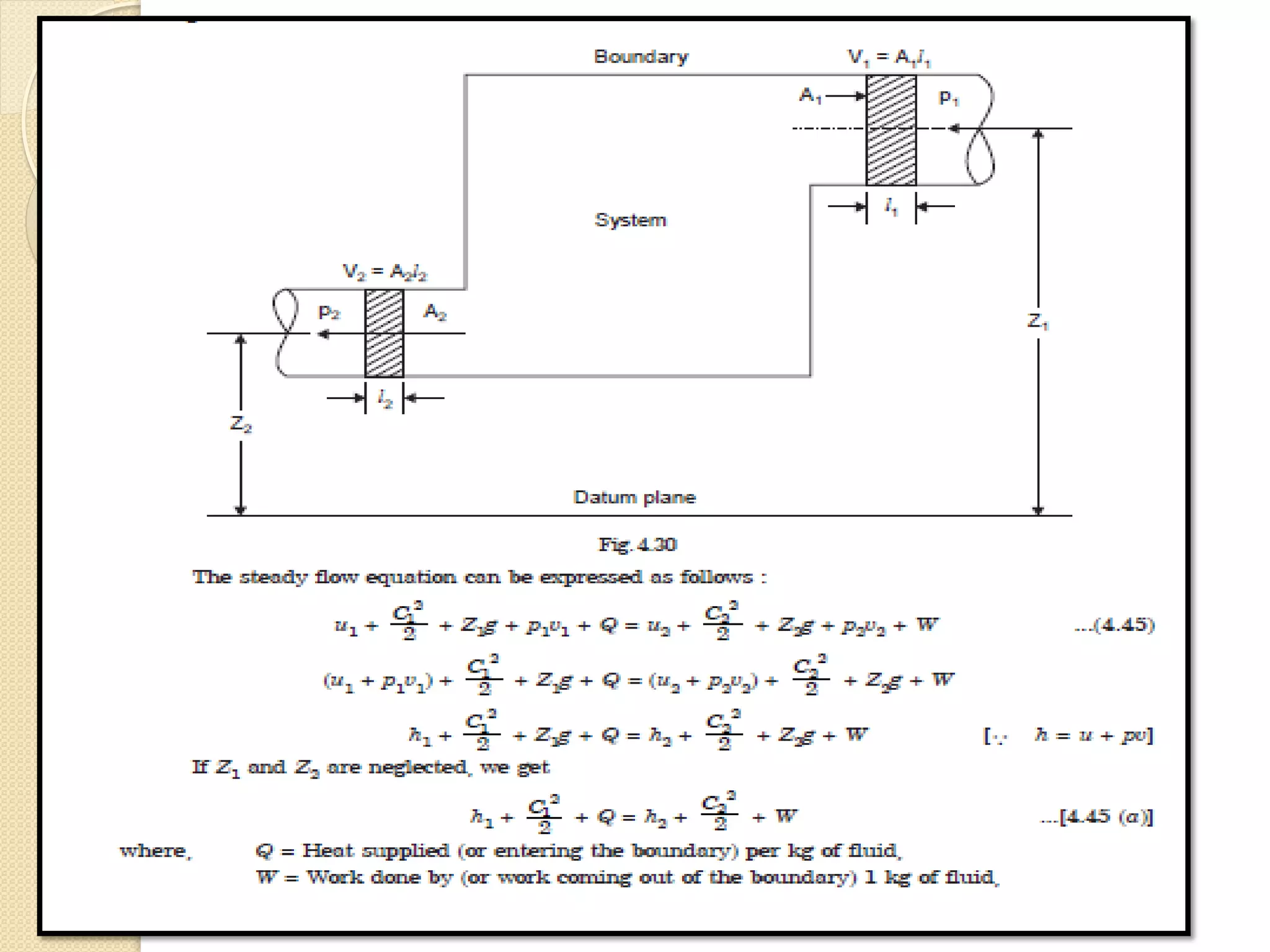
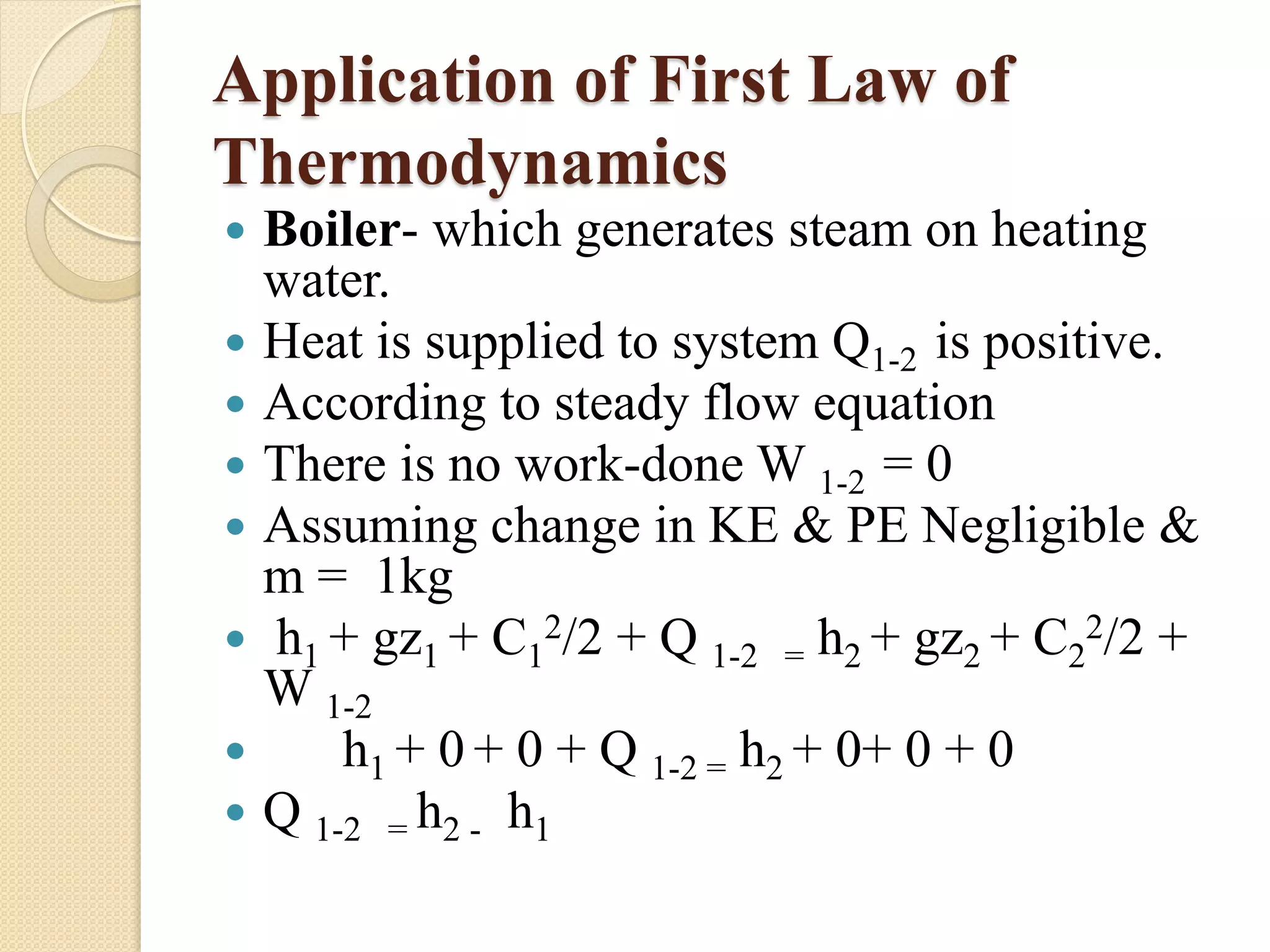
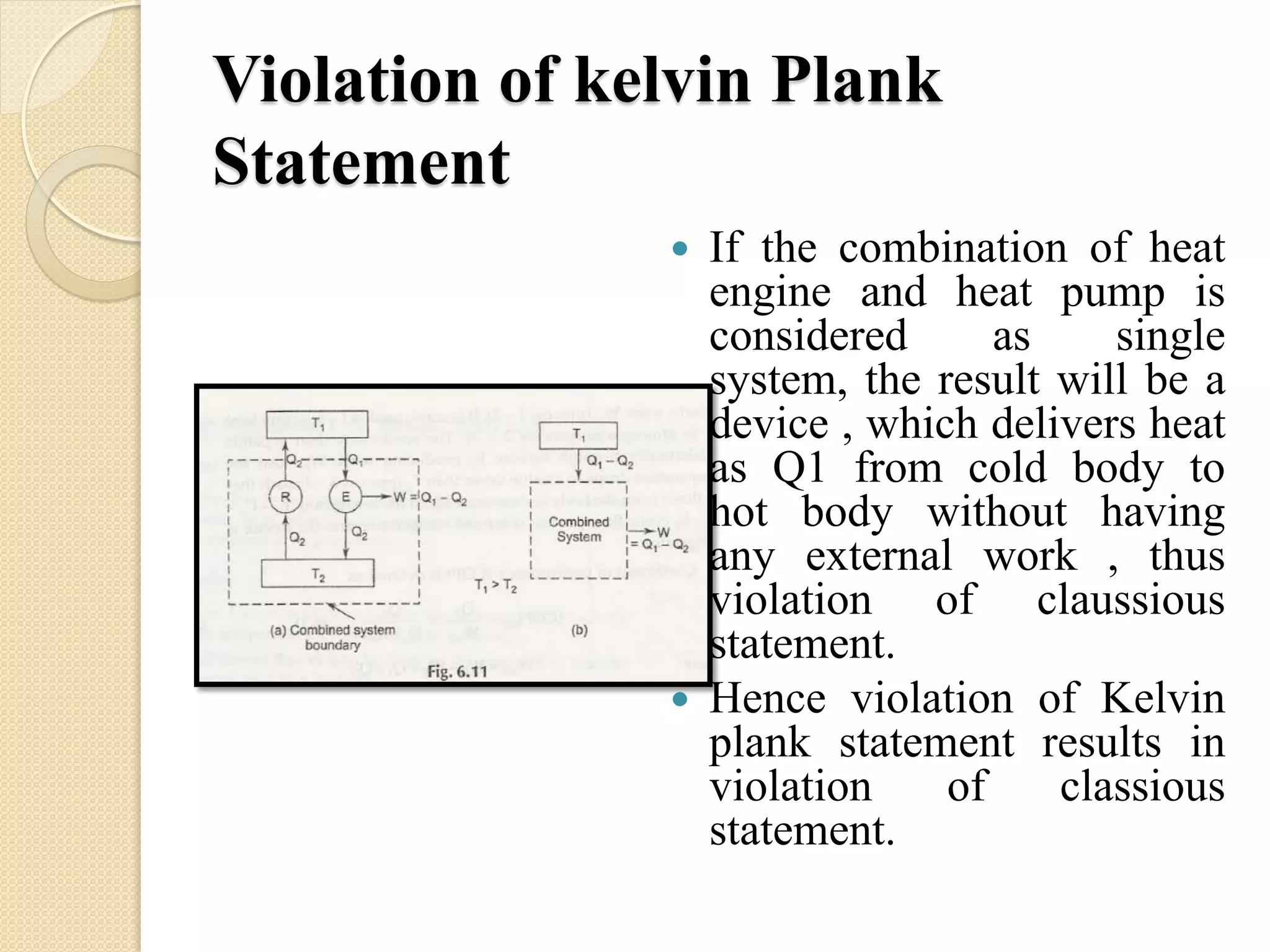
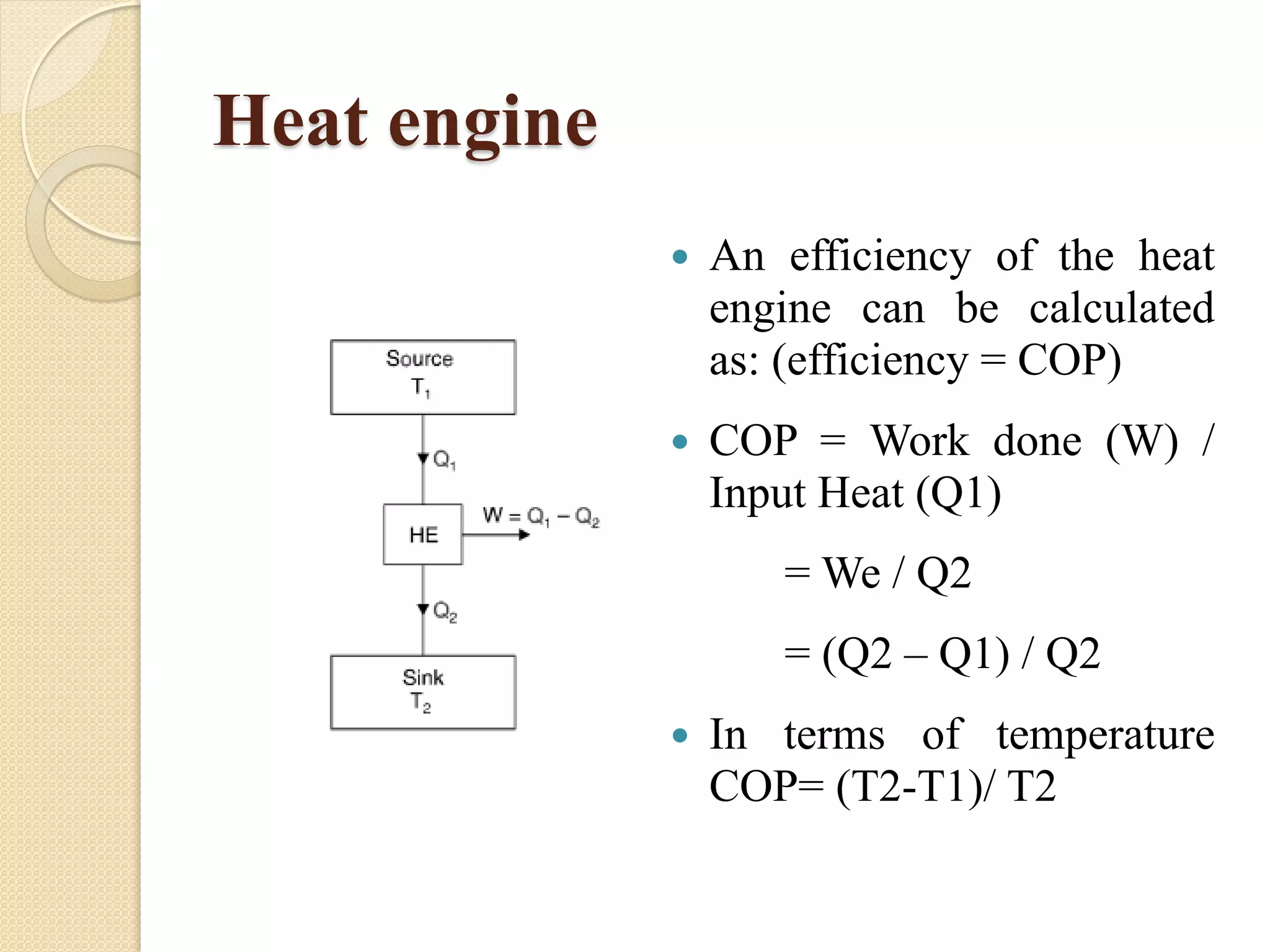
The document provides information on various thermodynamics concepts:
- A pure substance has a constant composition, while a mixture consists of multiple substances.
- A system is the quantity of matter under analysis, and can be open, closed, or isolated based on mass and energy transfers.
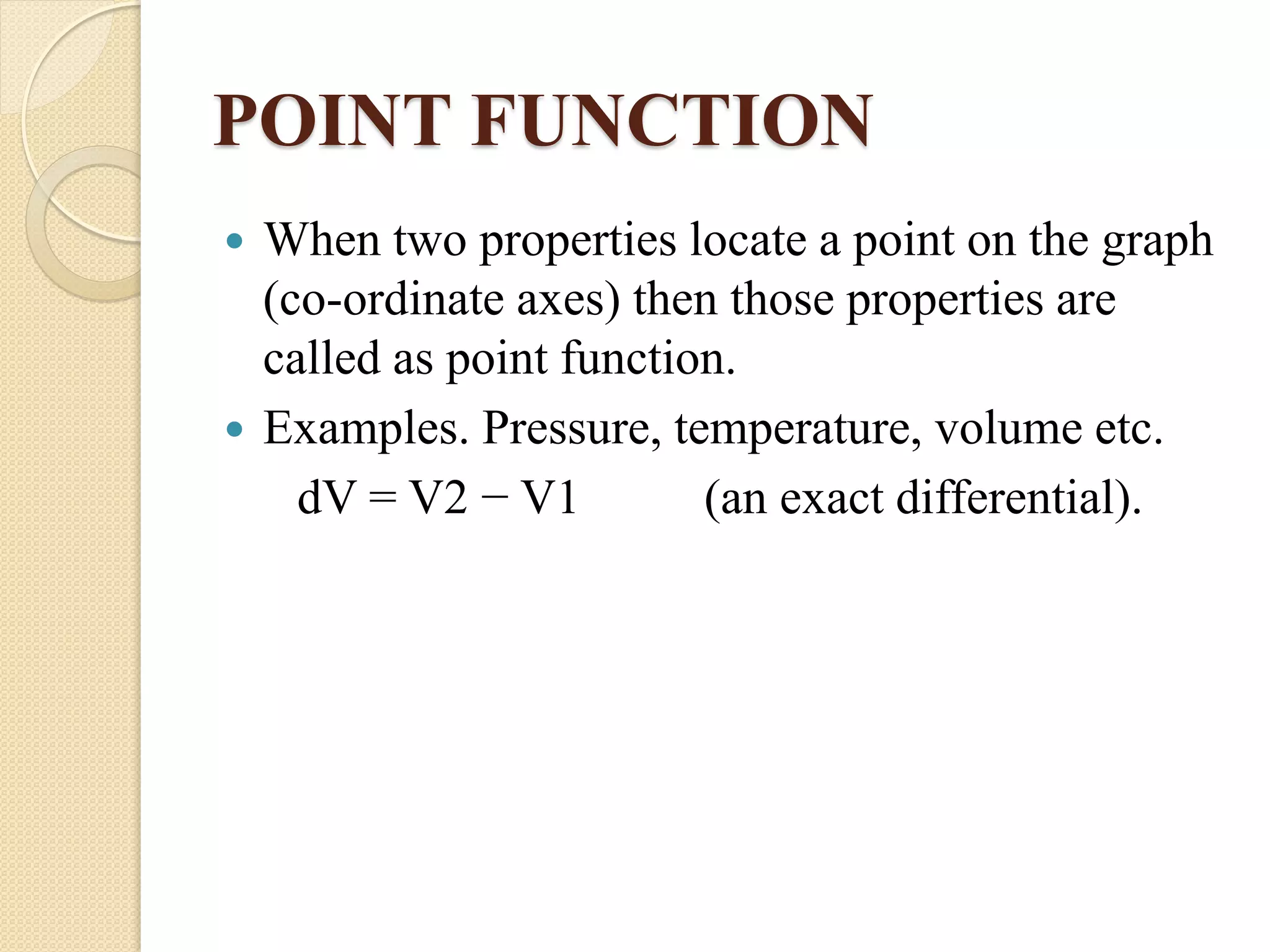
- Thermodynamic properties include intensive properties like temperature and pressure, and extensive properties like volume and energy which depend on system mass.
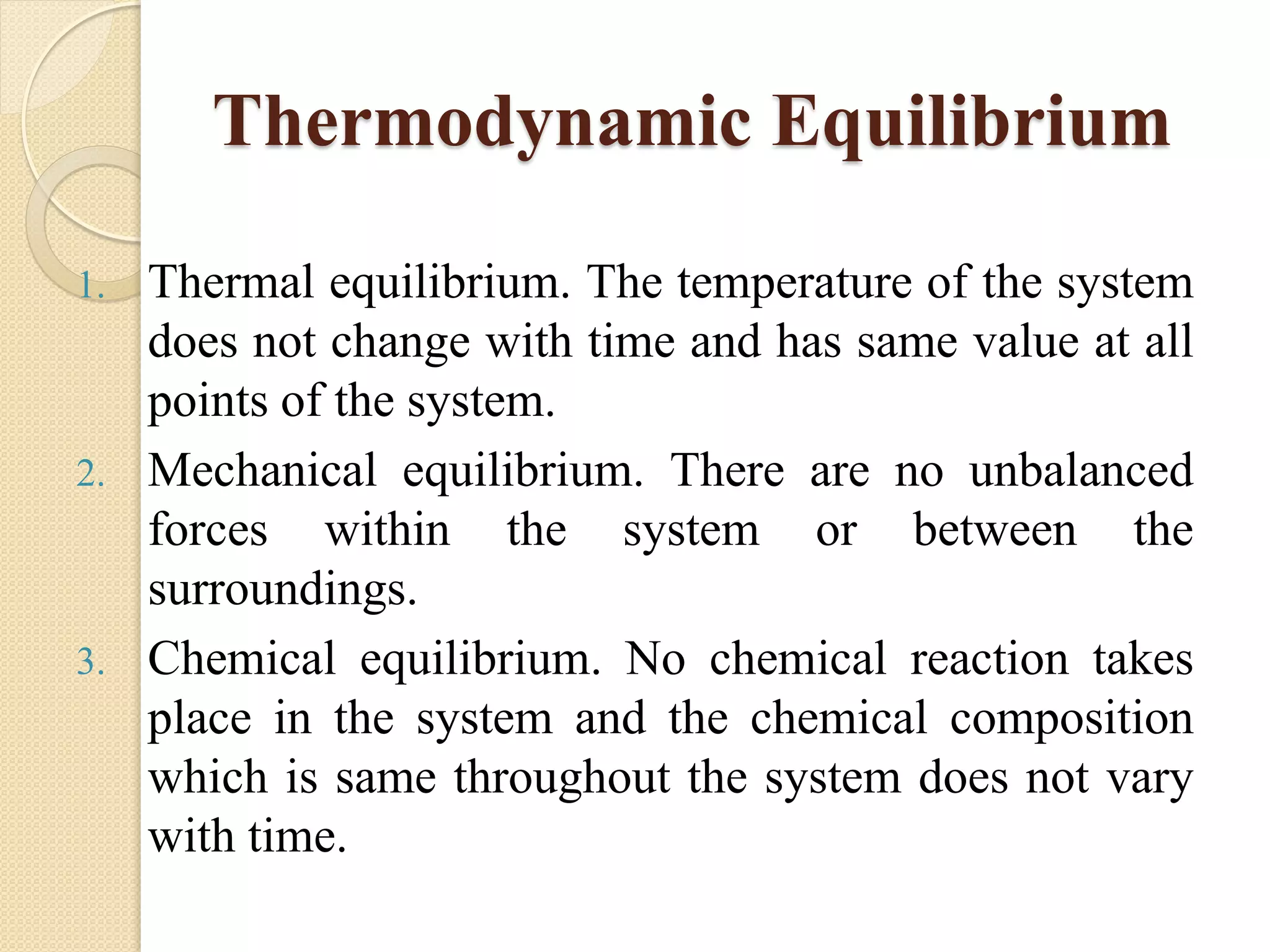
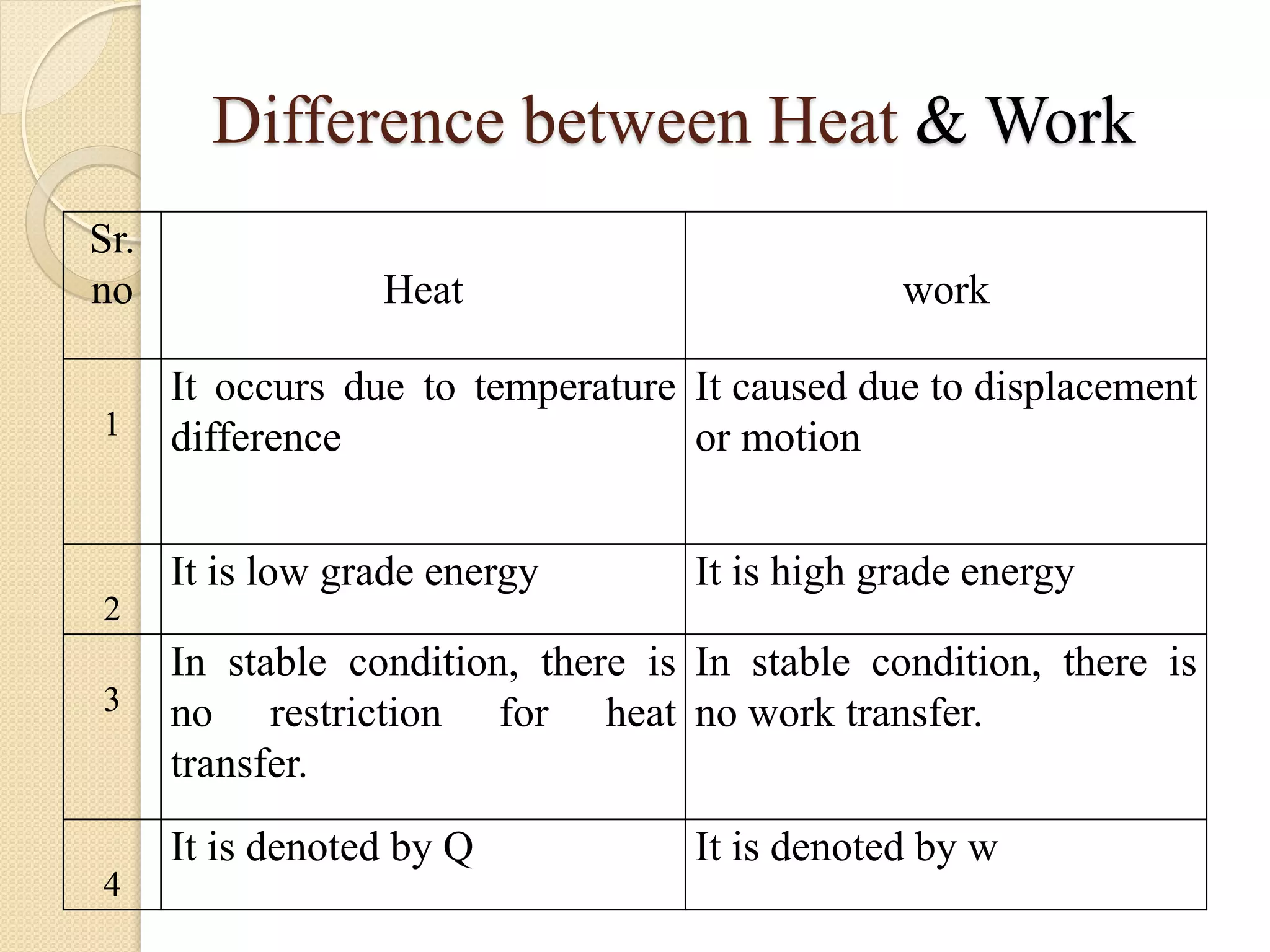
- Processes involve system state changes or energy transfers. Equilibrium occurs when properties are uniform throughout the system.