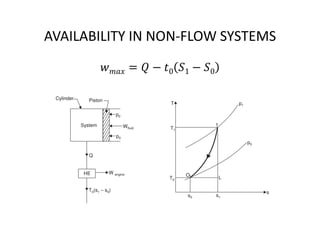
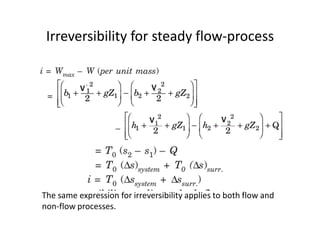
This document discusses availability and irreversibility in transient and steady flow systems. It provides definitions for available energy, unavailable energy, dead state, and effectiveness. It examines availability in terms of the first and second laws of thermodynamics. Equations are given for calculating maximum work, availability, and irreversibility for various thermodynamic processes involving changes in temperature, pressure, and volume. Two sample problems are included and solved to demonstrate calculating work, availability change, and irreversibility for specified thermodynamic systems.