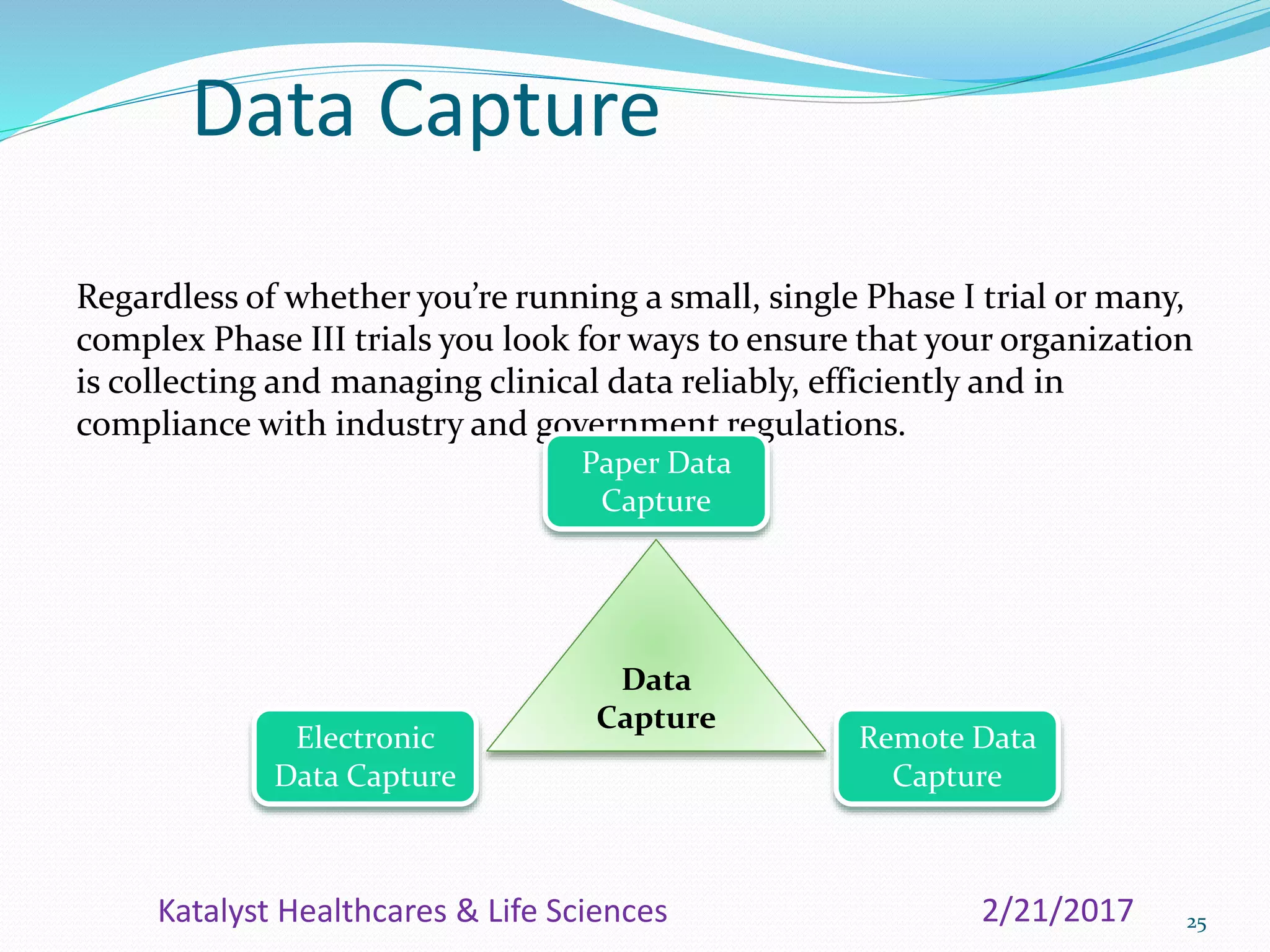
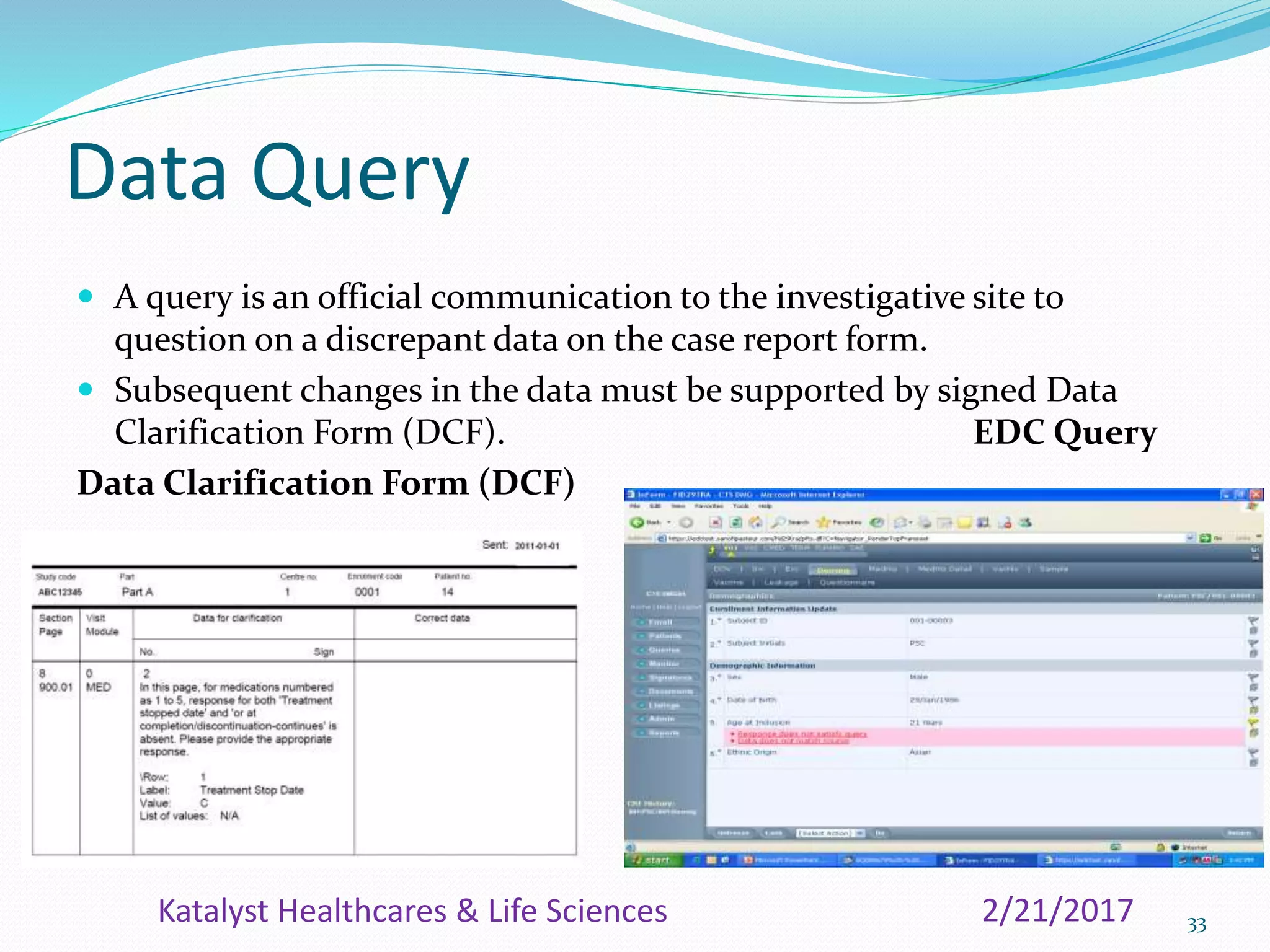
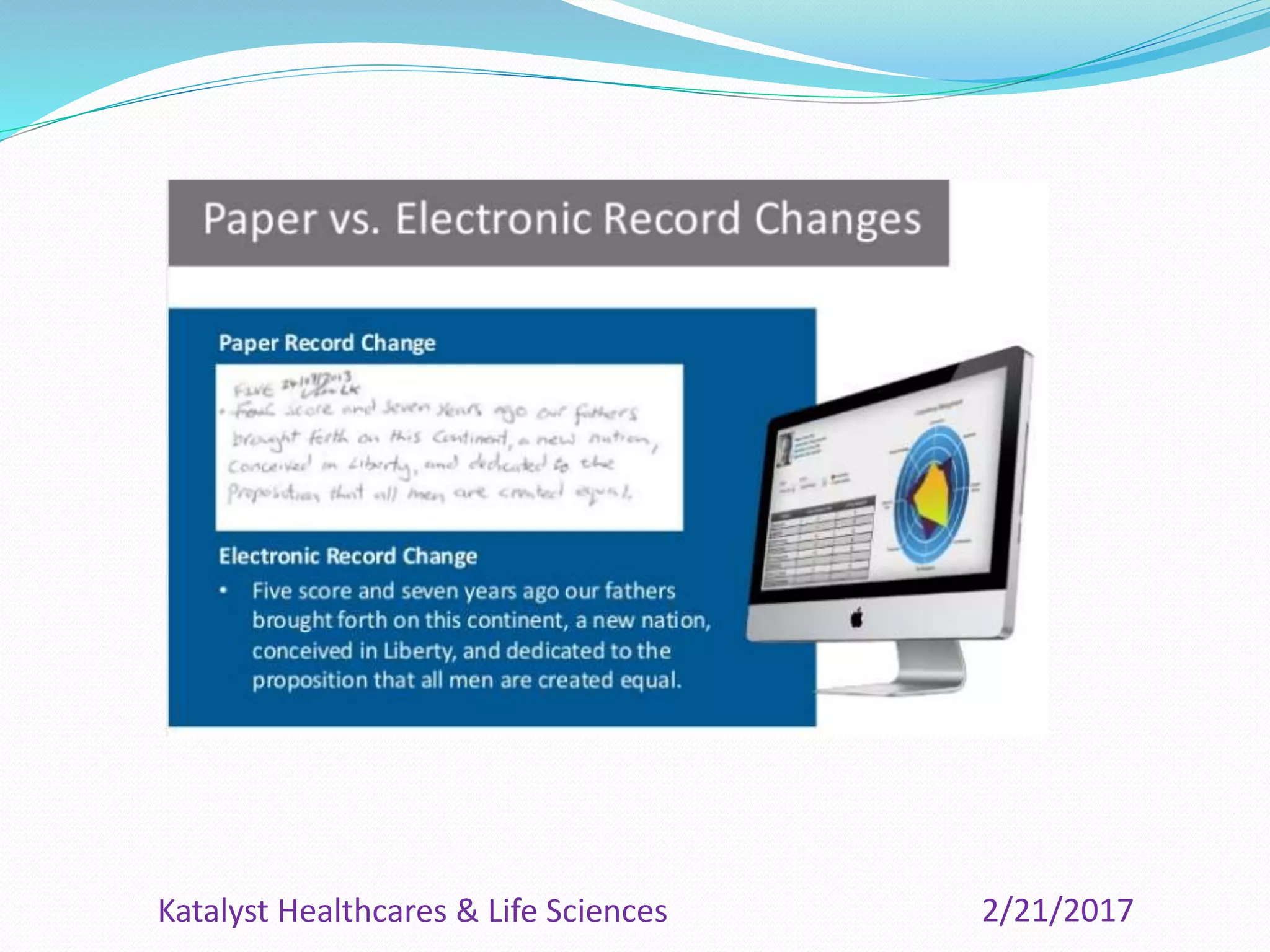
The document provides an overview of the Clinical Data Management (CDM) process, detailing its role in the entry, verification, and validation of data collected during clinical trials. It emphasizes the importance of maintaining data integrity for drug approval by regulatory agencies and outlines the various phases of clinical trials and the multidisciplinary roles involved. Additionally, it covers the objectives, activities, and significance of CDM, including data collection methods and quality assurance practices.