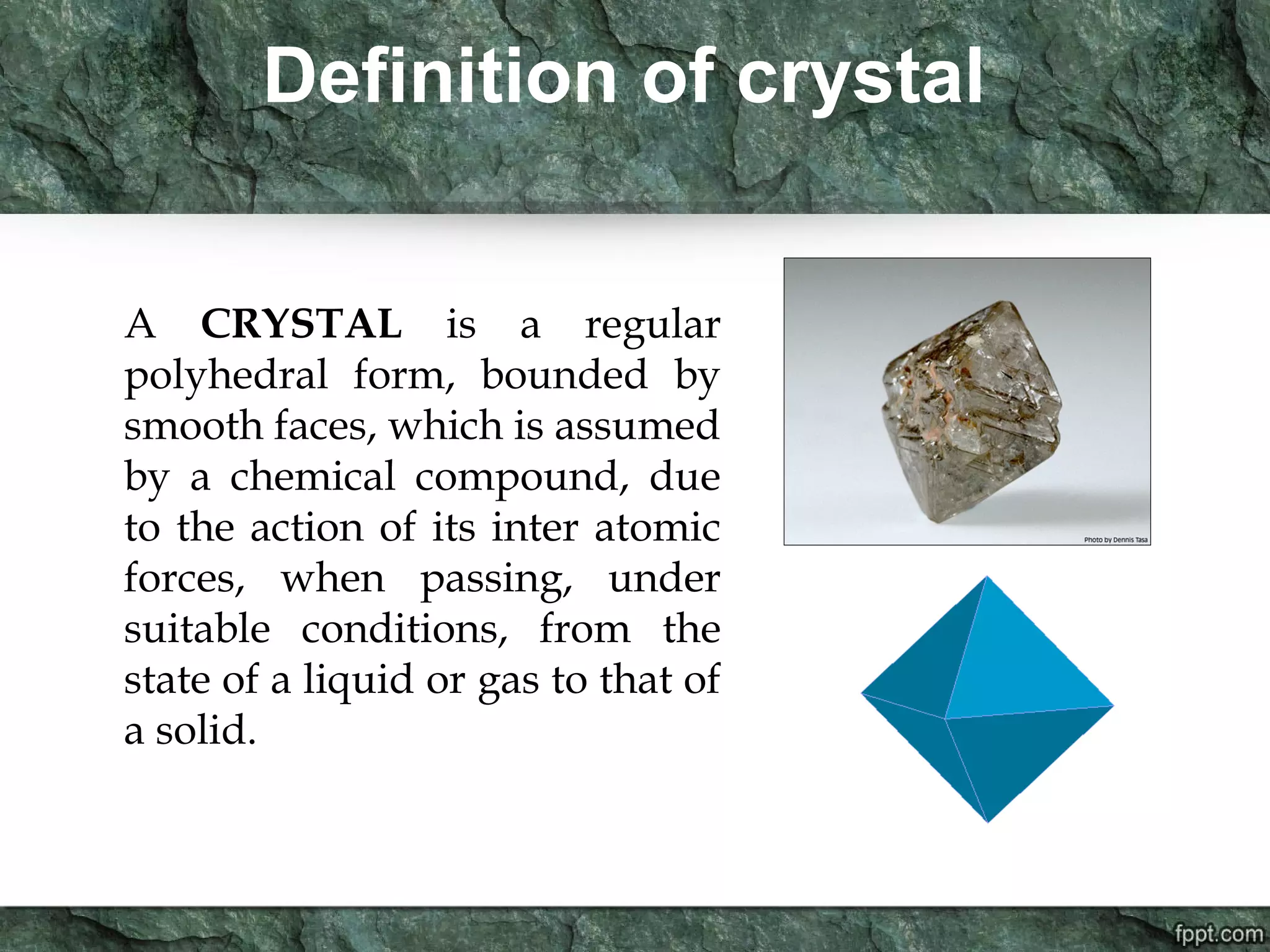
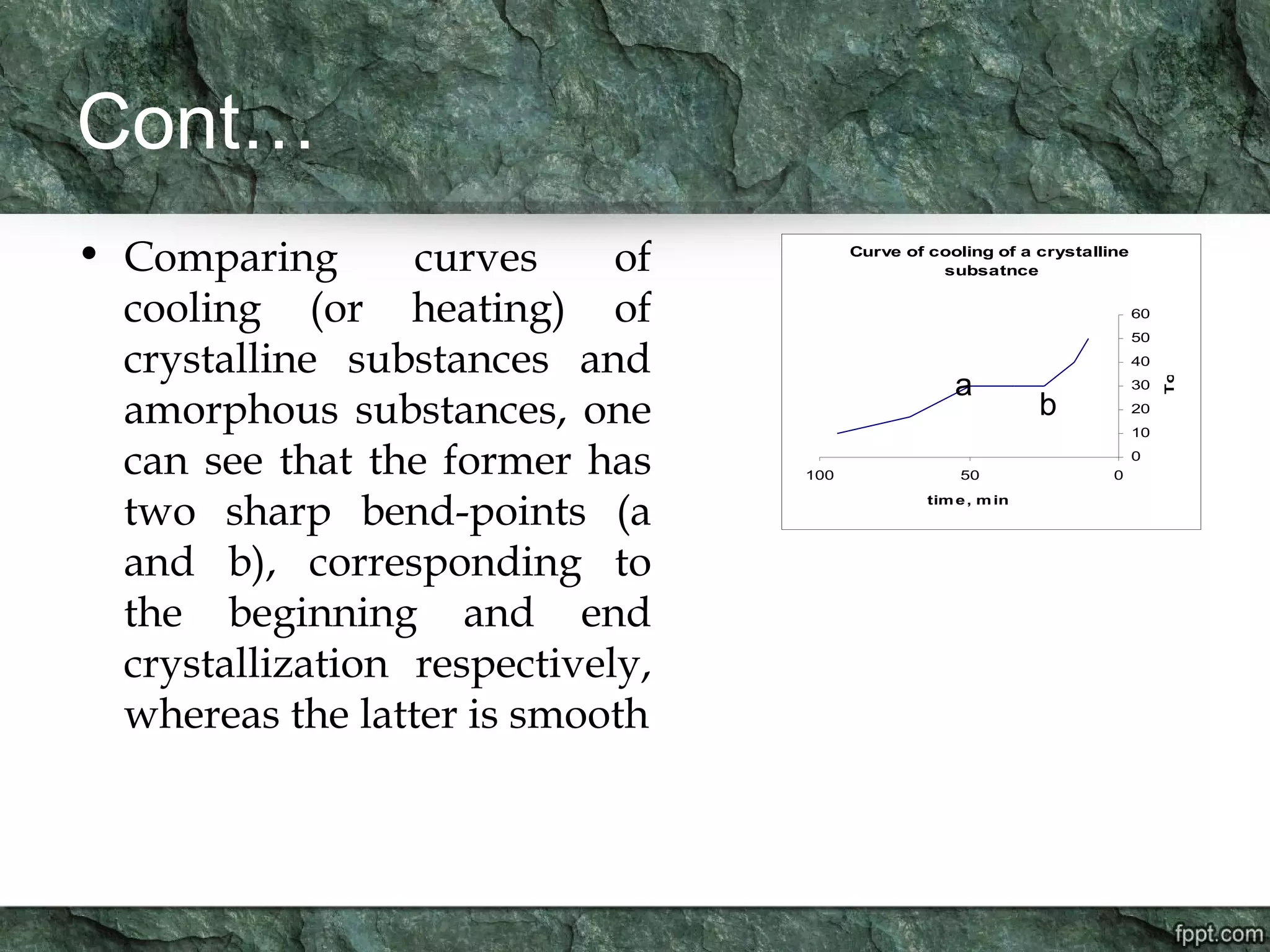
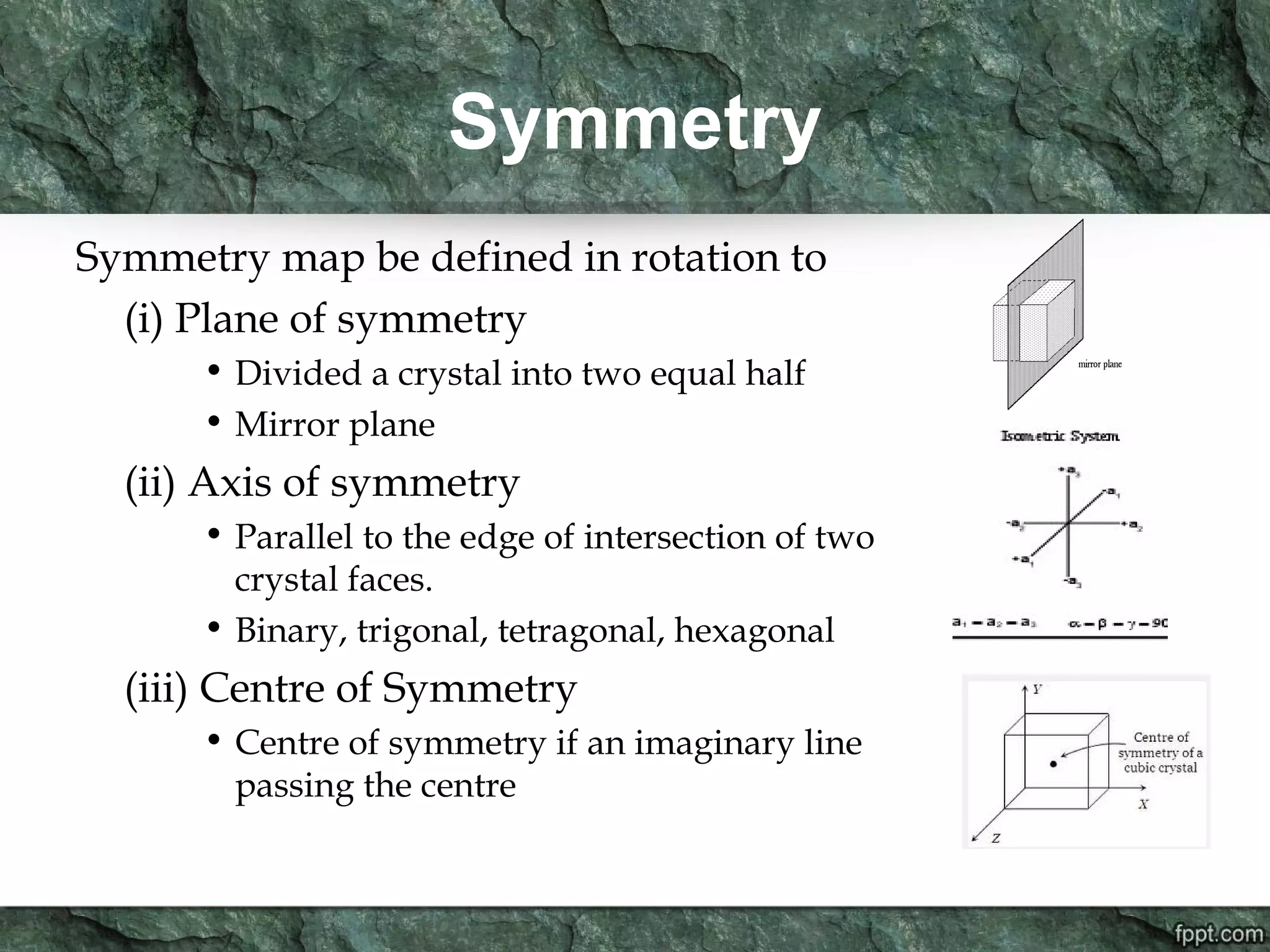
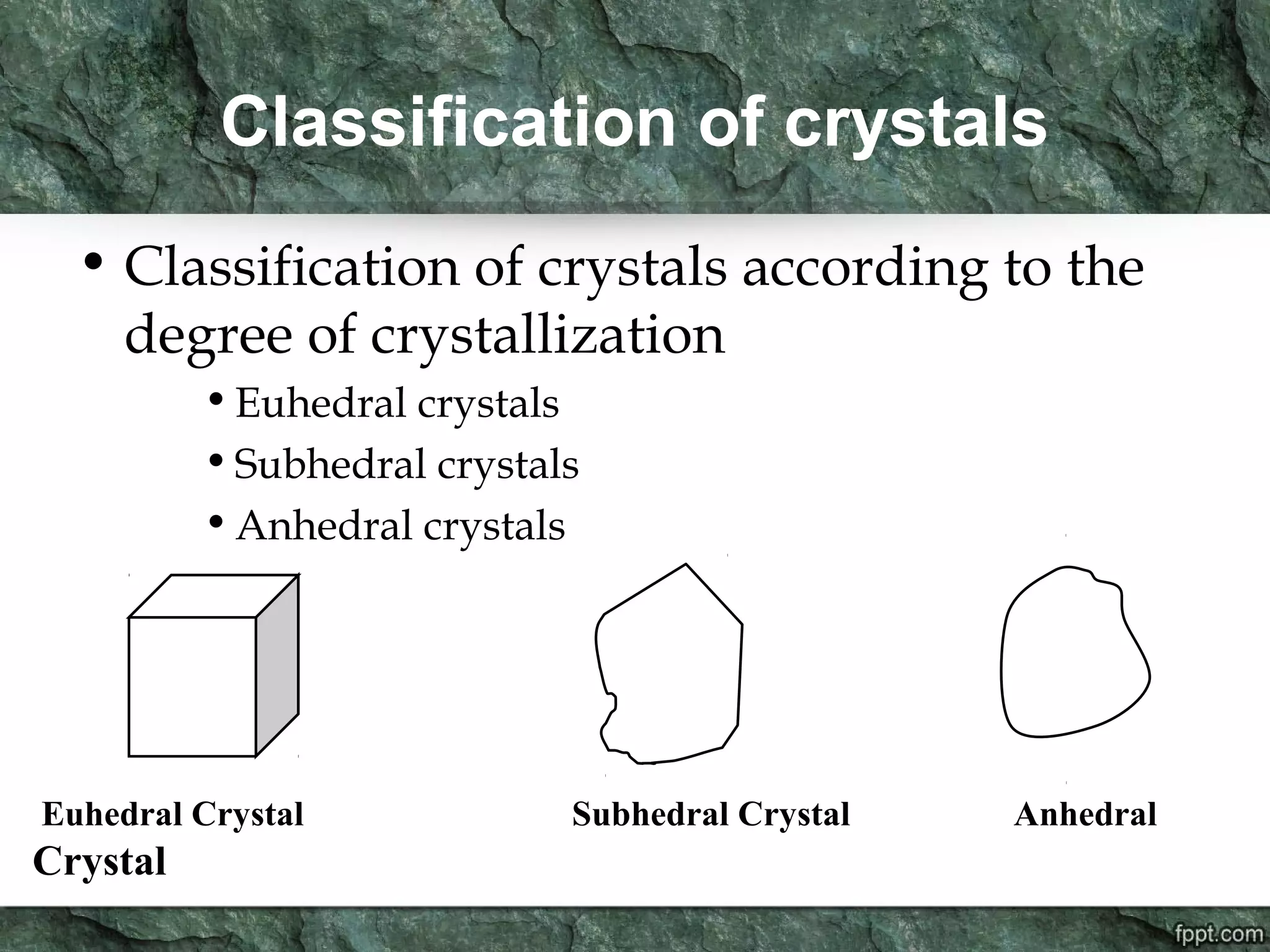
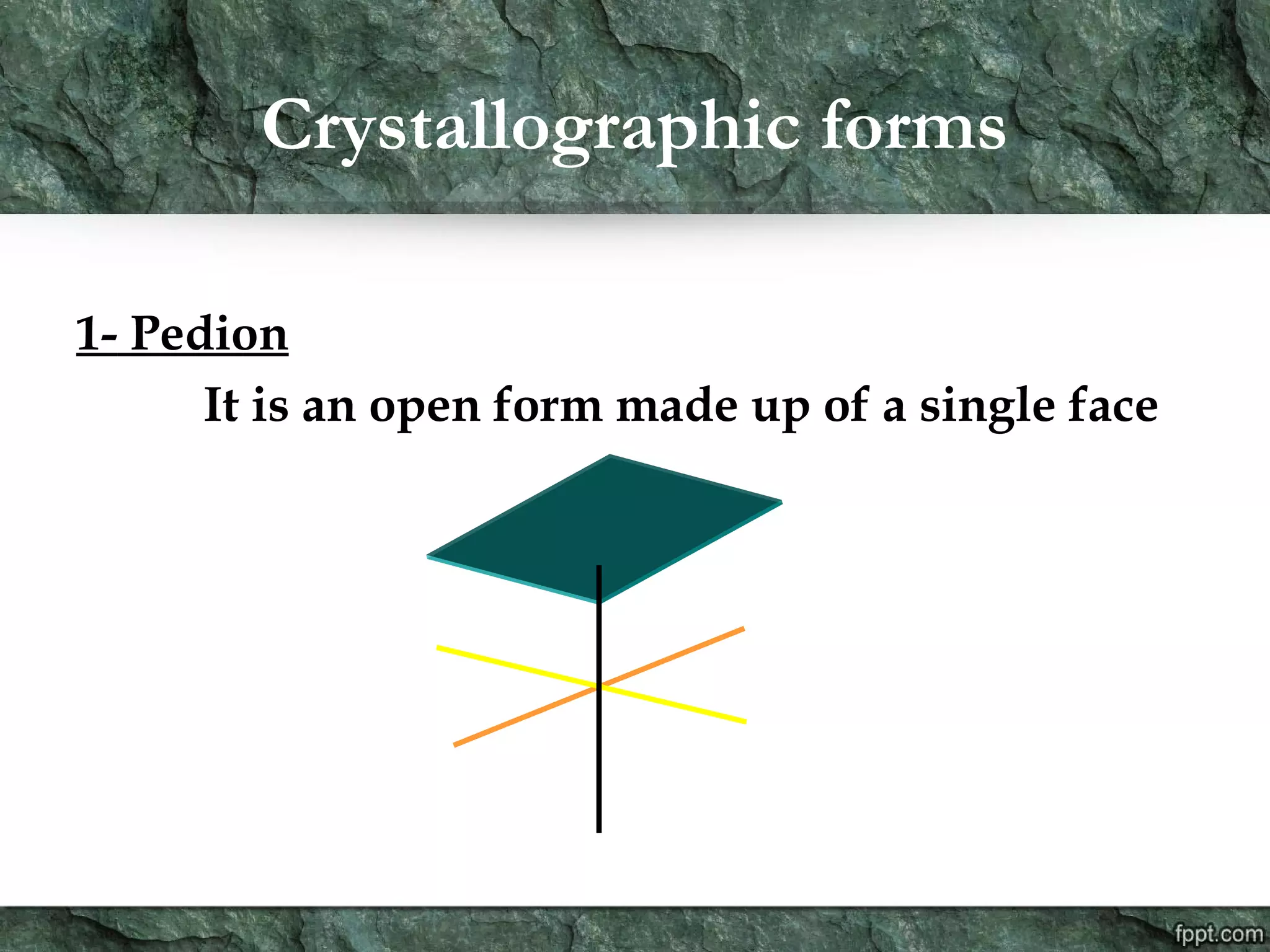
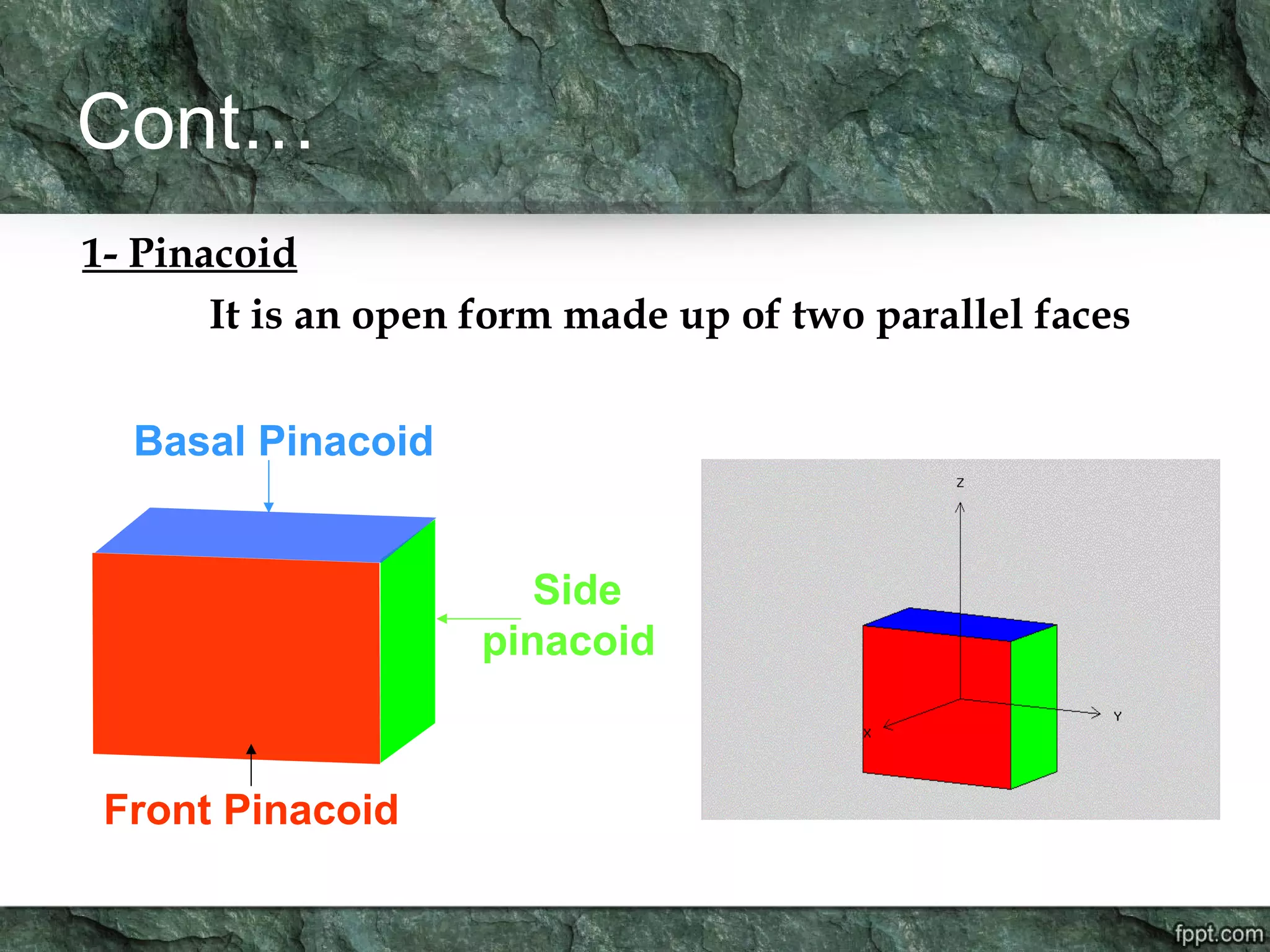
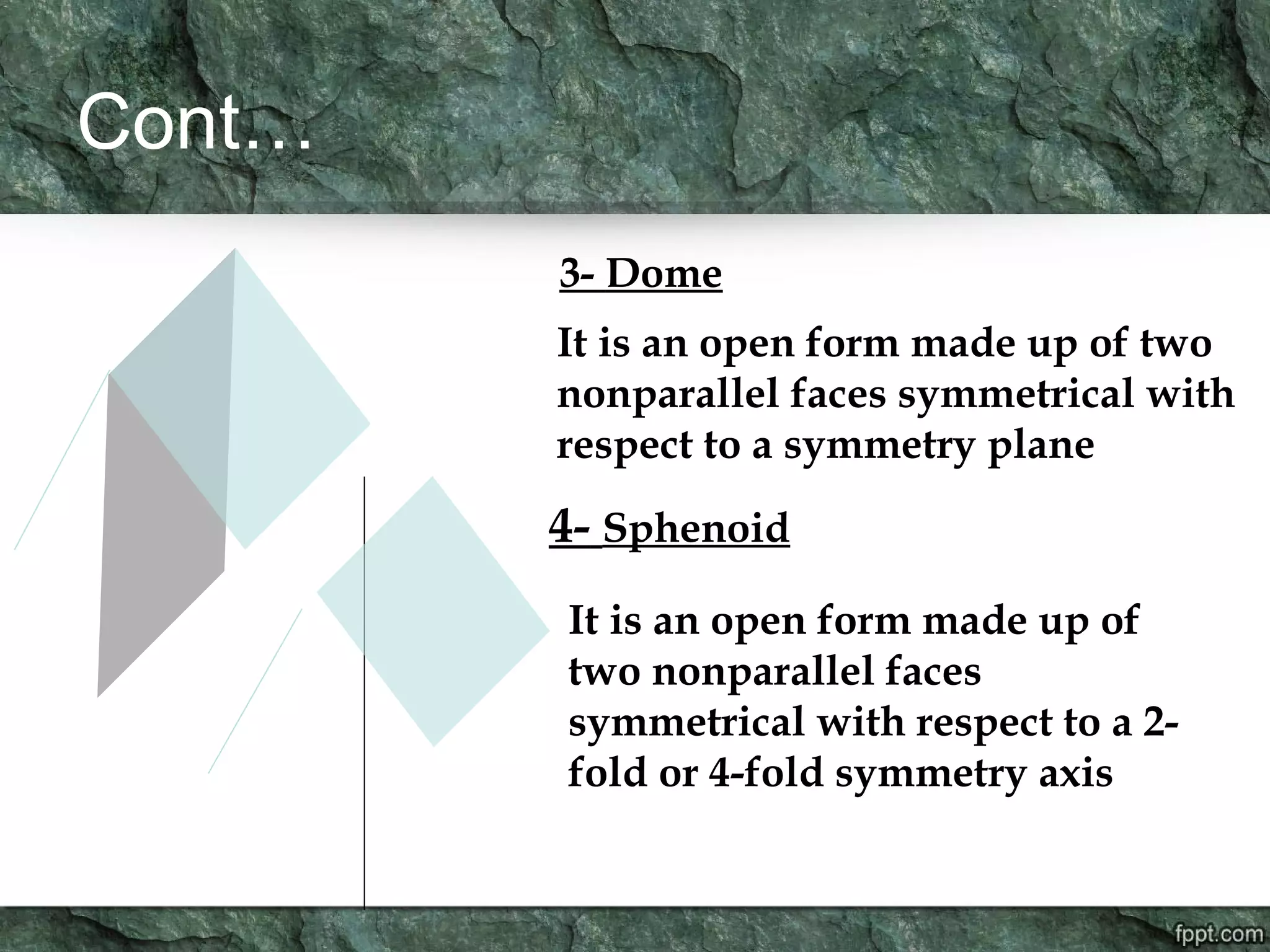
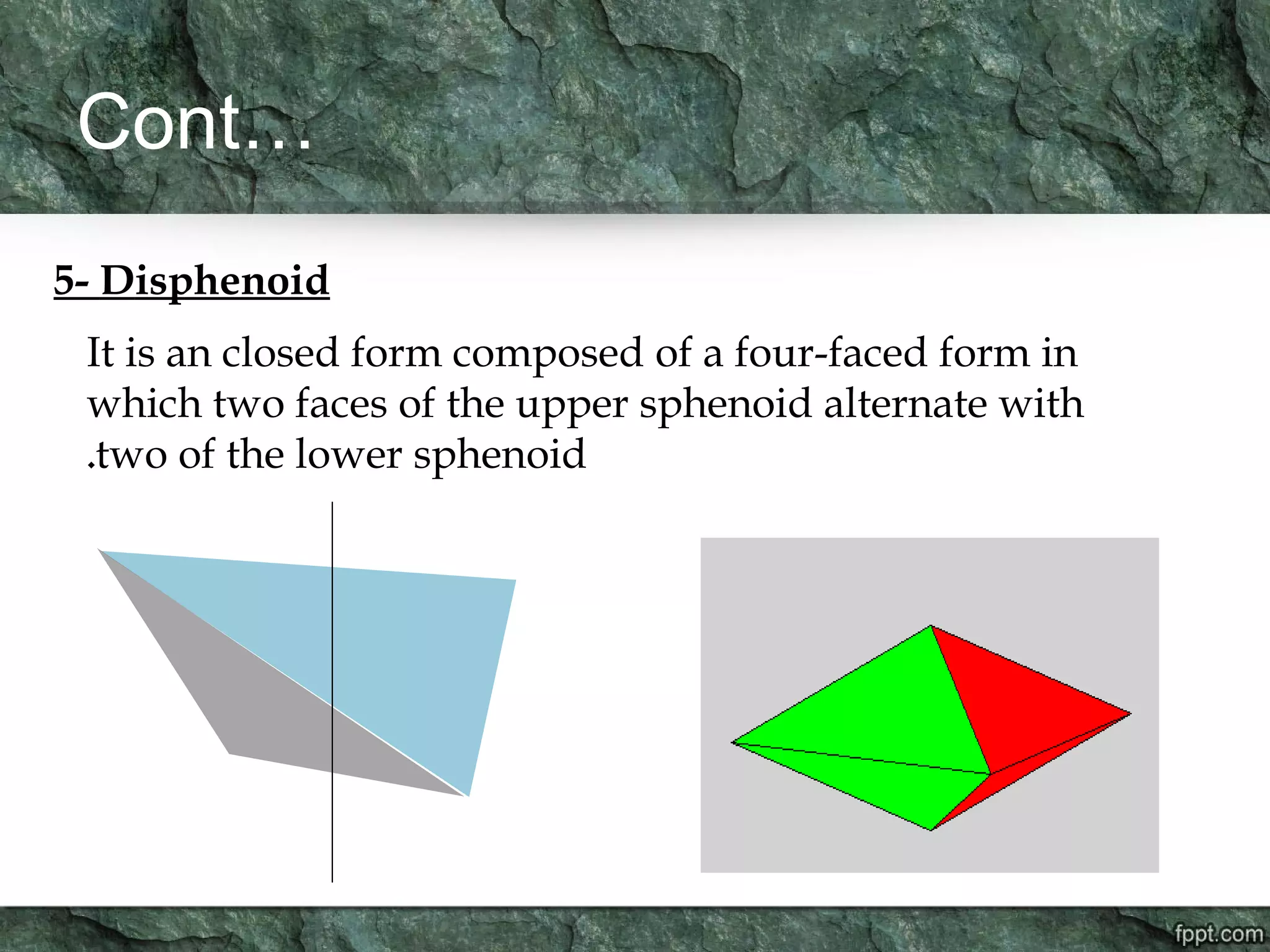
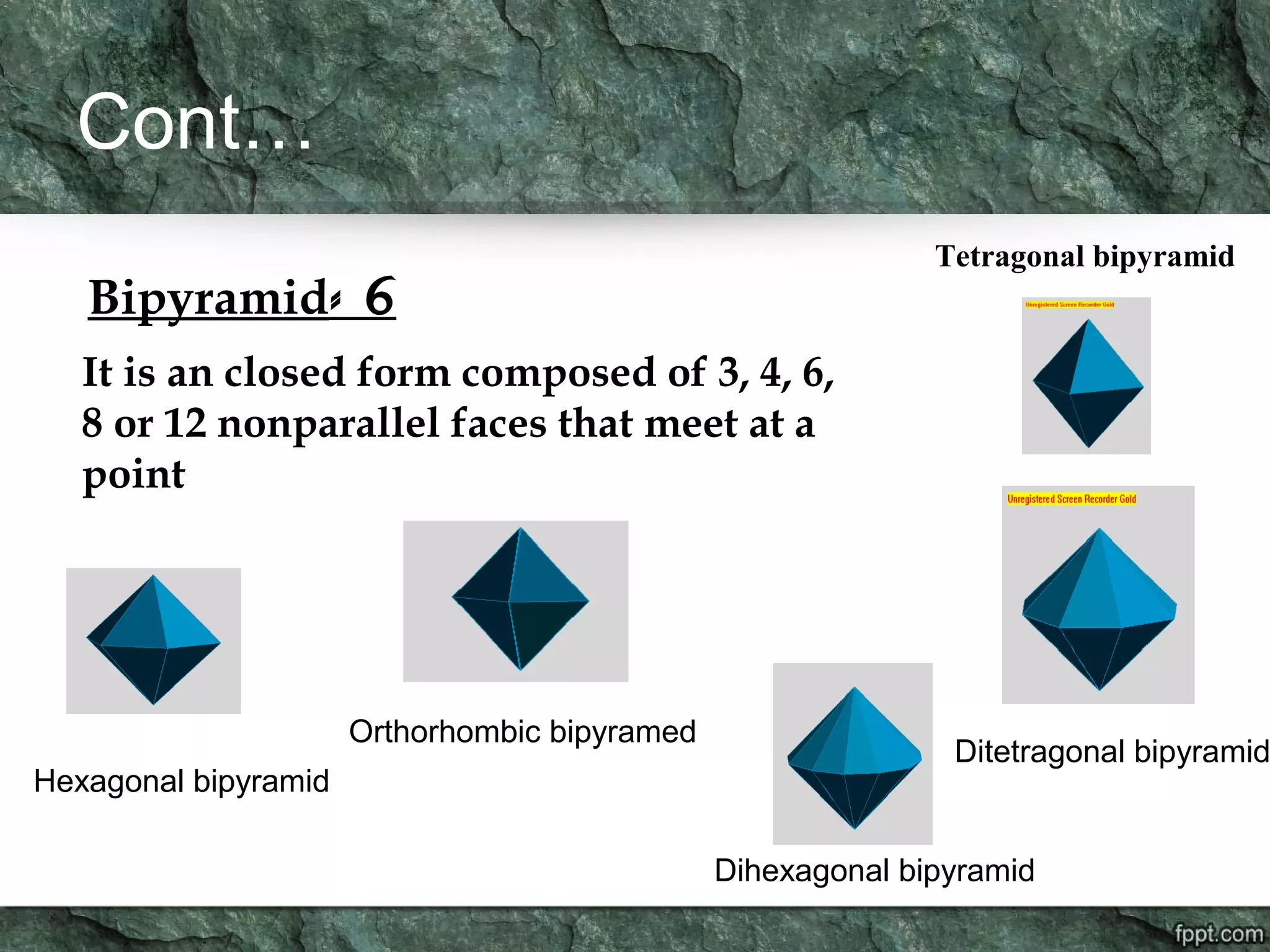
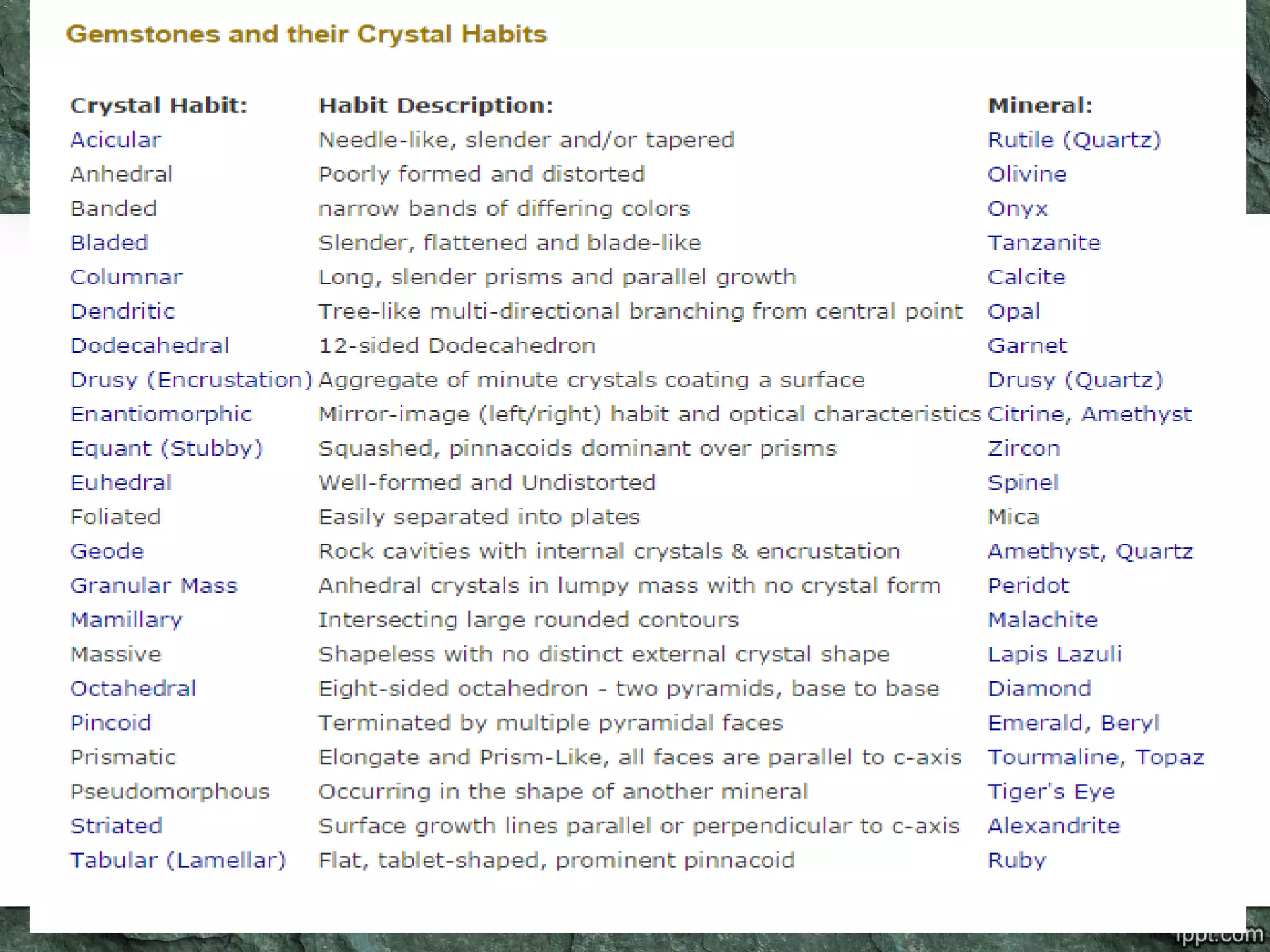
Crystallography is the study of crystals, their growth, structure, and properties. A crystal is a solid with a regular, repeating internal structure at the atomic scale. Crystals have long-range order and are bounded by smooth, flat faces. Crystals can be classified based on their degree of crystallinity as euhedral, subhedral, or anhedral. Crystals exhibit symmetry properties like planes, axes, and centers of symmetry. They can also take on specific crystallographic forms like prisms, bipyramids, and rhombohedra. A crystal's habit describes its external shape and is influenced by its structure and growth conditions like temperature, pressure, and impurities.