The document discusses several topics related to crystals:
- Crystals are solid structures composed of periodic arrangements of atoms that can form regular geometric shapes. Common crystal shapes include cubic, hexagonal, and tetragonal.
- Crystals exhibit symmetry properties including centers of symmetry, planes of symmetry, and axes of symmetry. These symmetries are used to classify crystals into seven crystal systems.
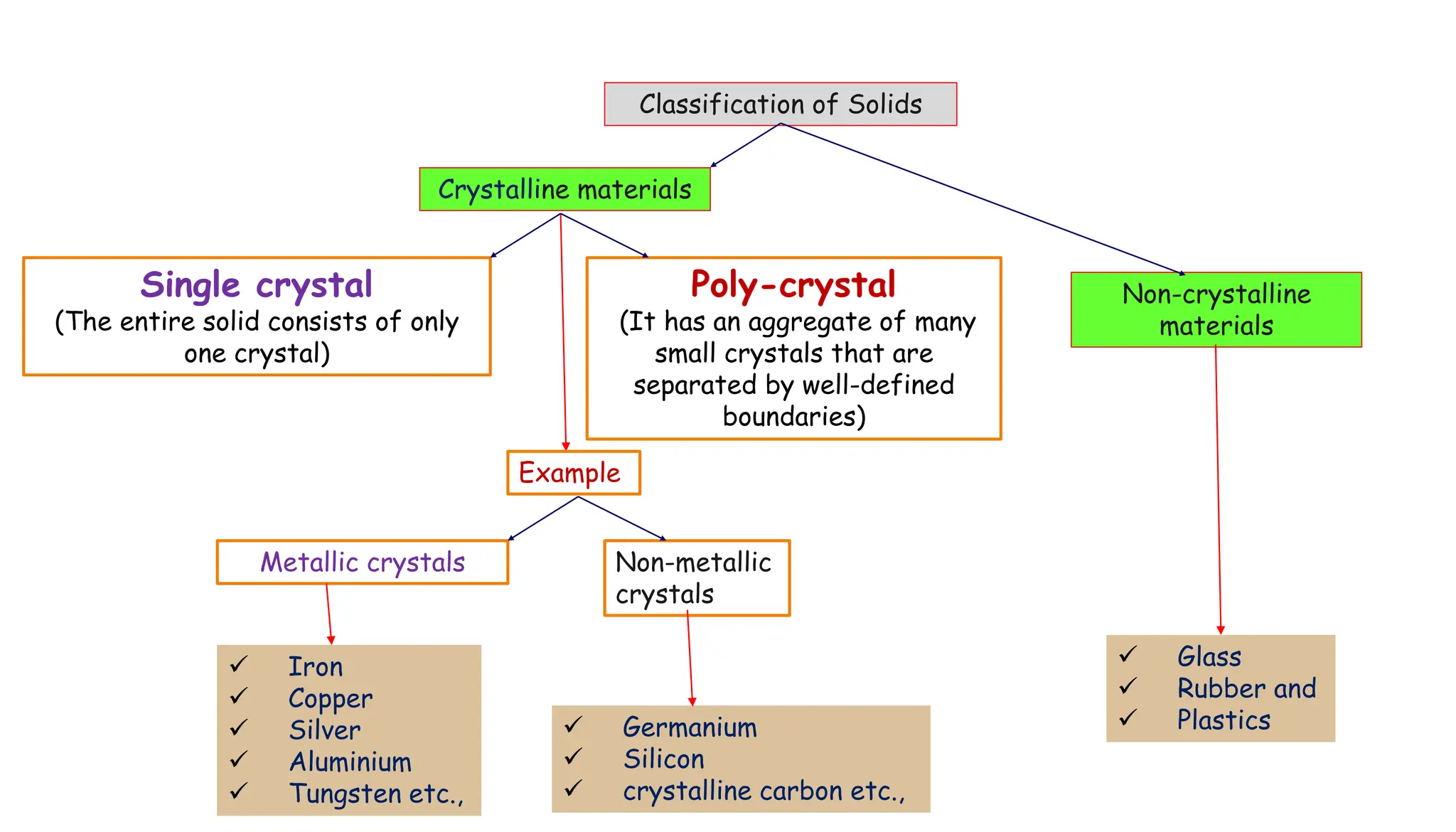
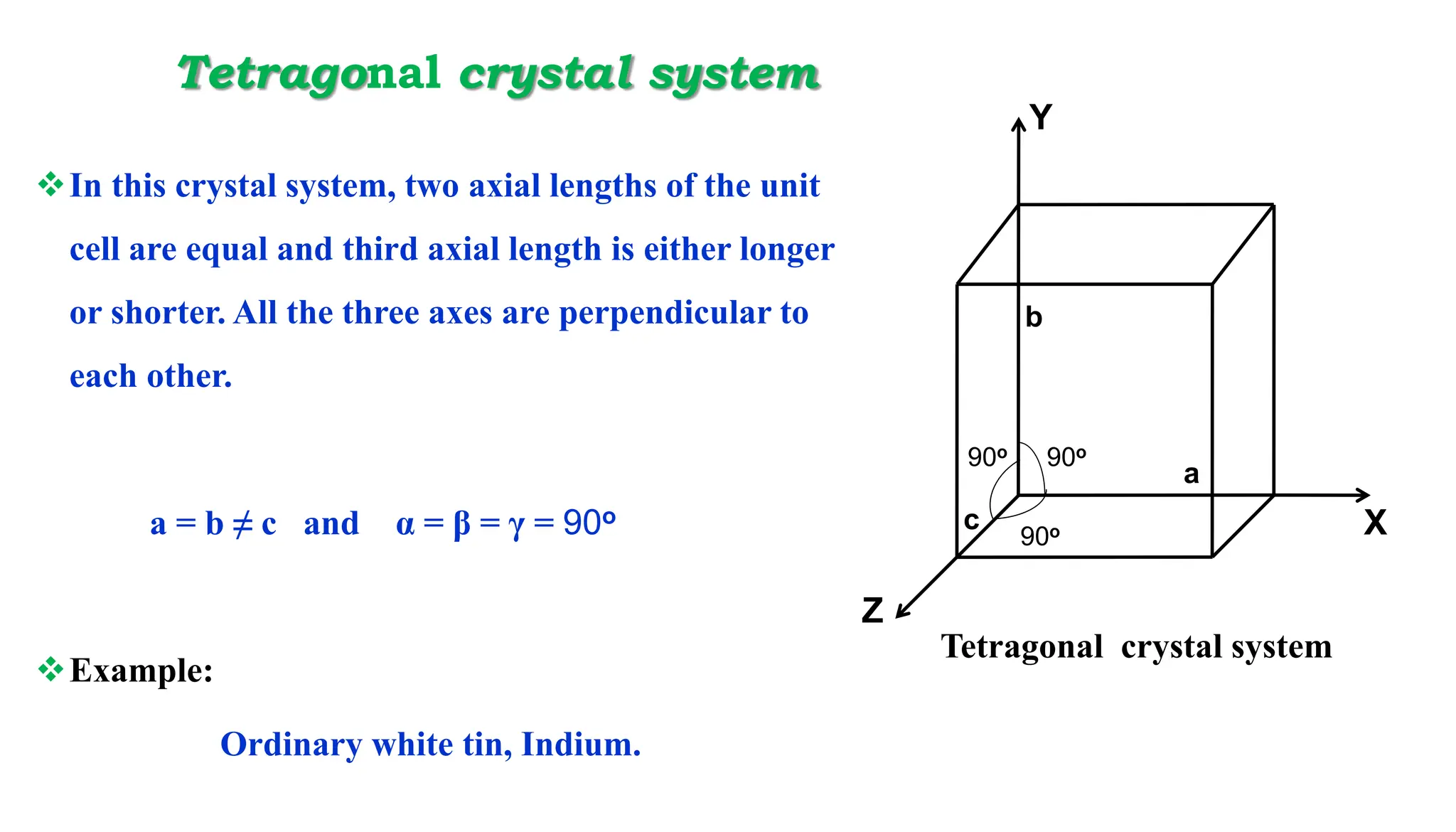
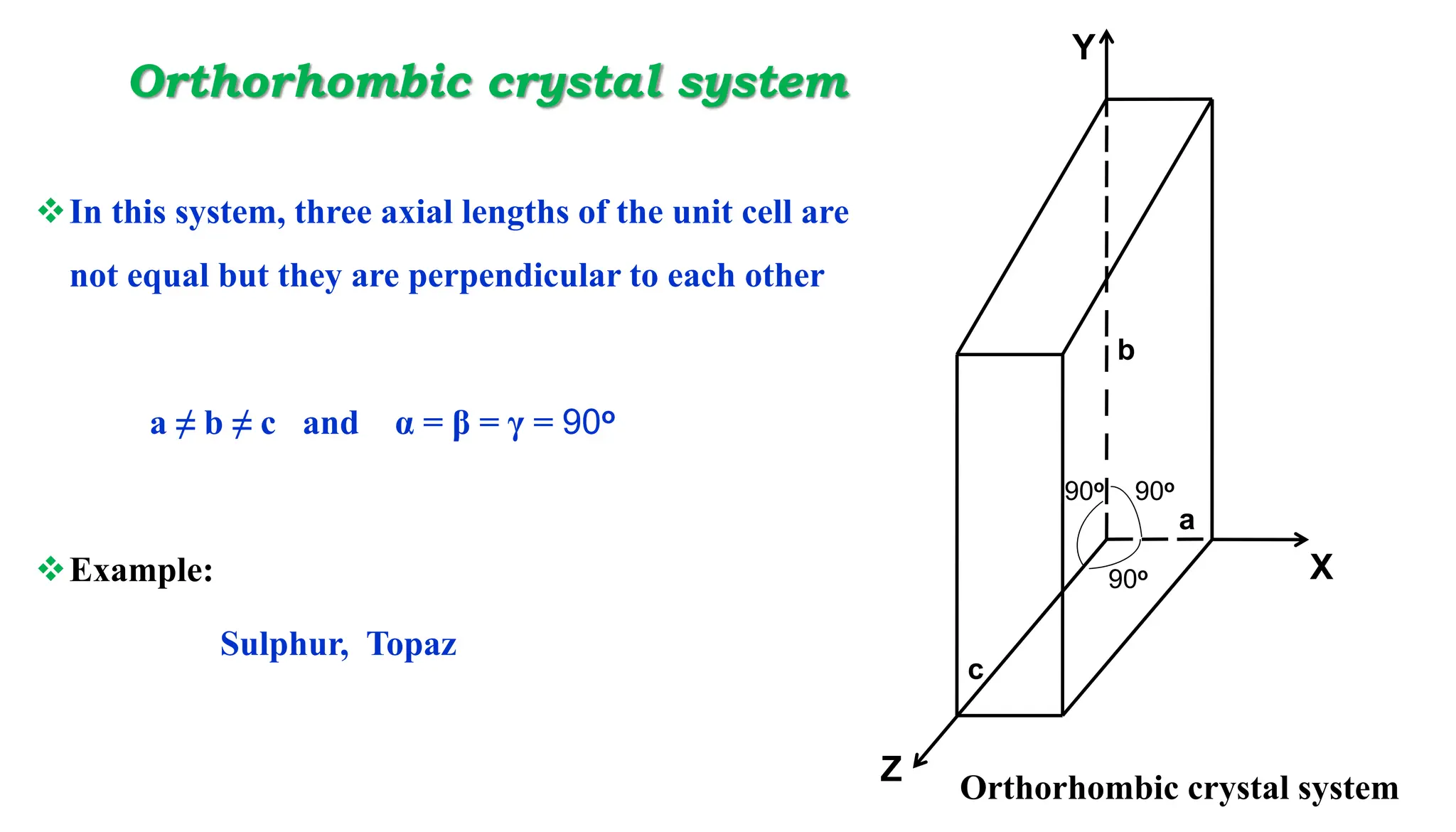
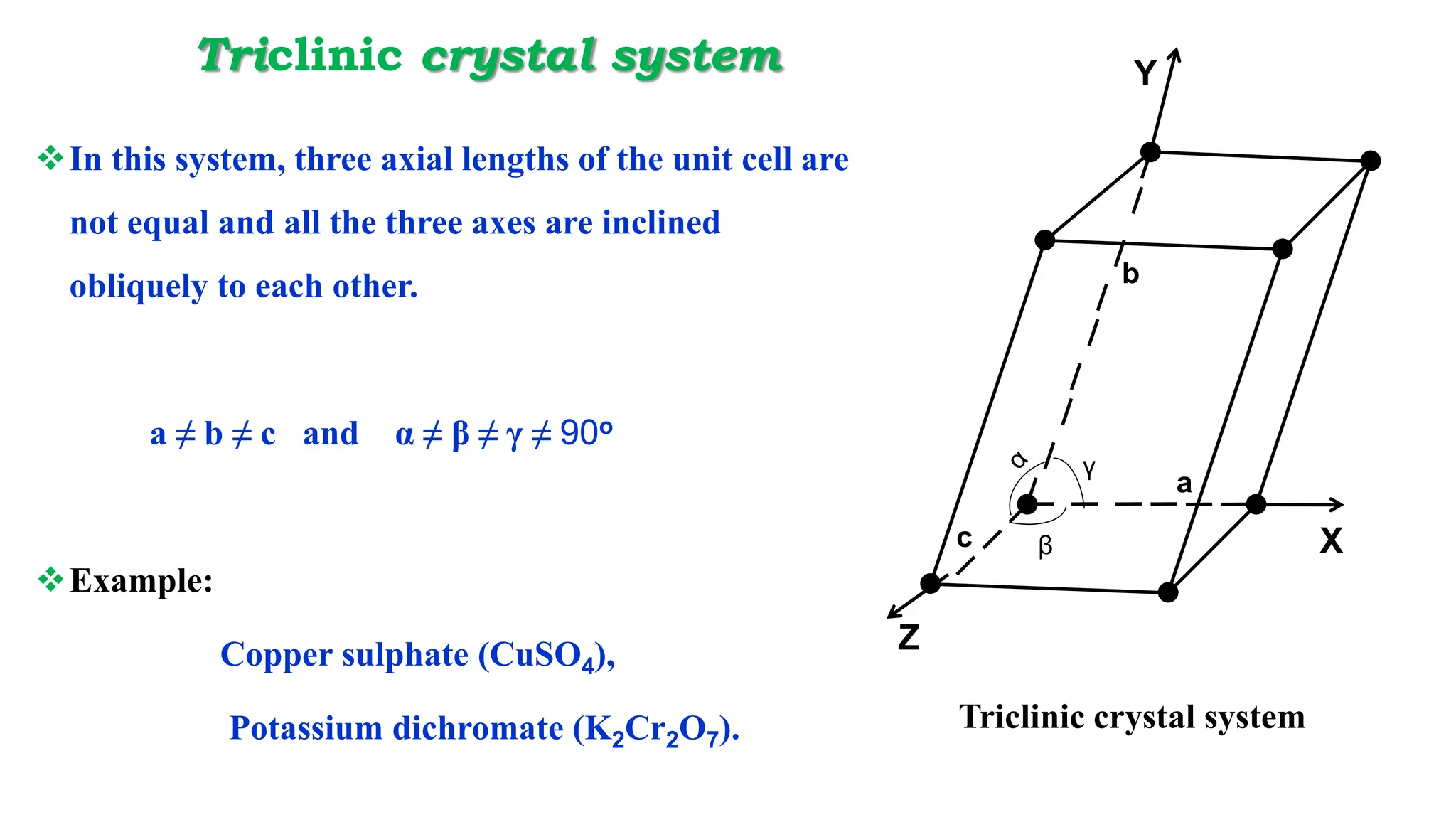
- Important crystal systems include cubic, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal. Unit cells define the basic repeating structure of crystals and can be primitive or non-primitive.
- Crystals are grown to specific sizes optimal for their intended use, such as 50μm