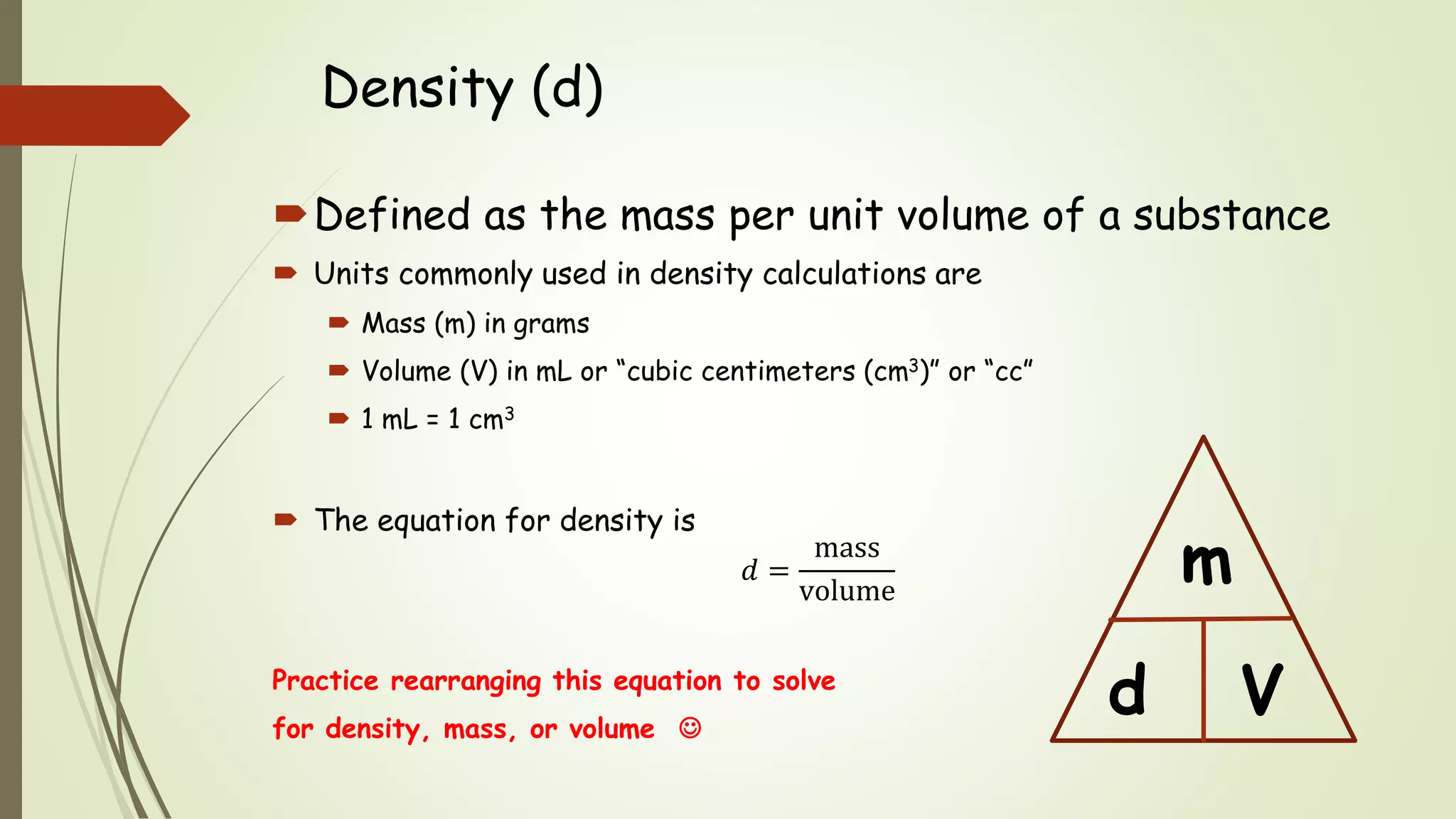
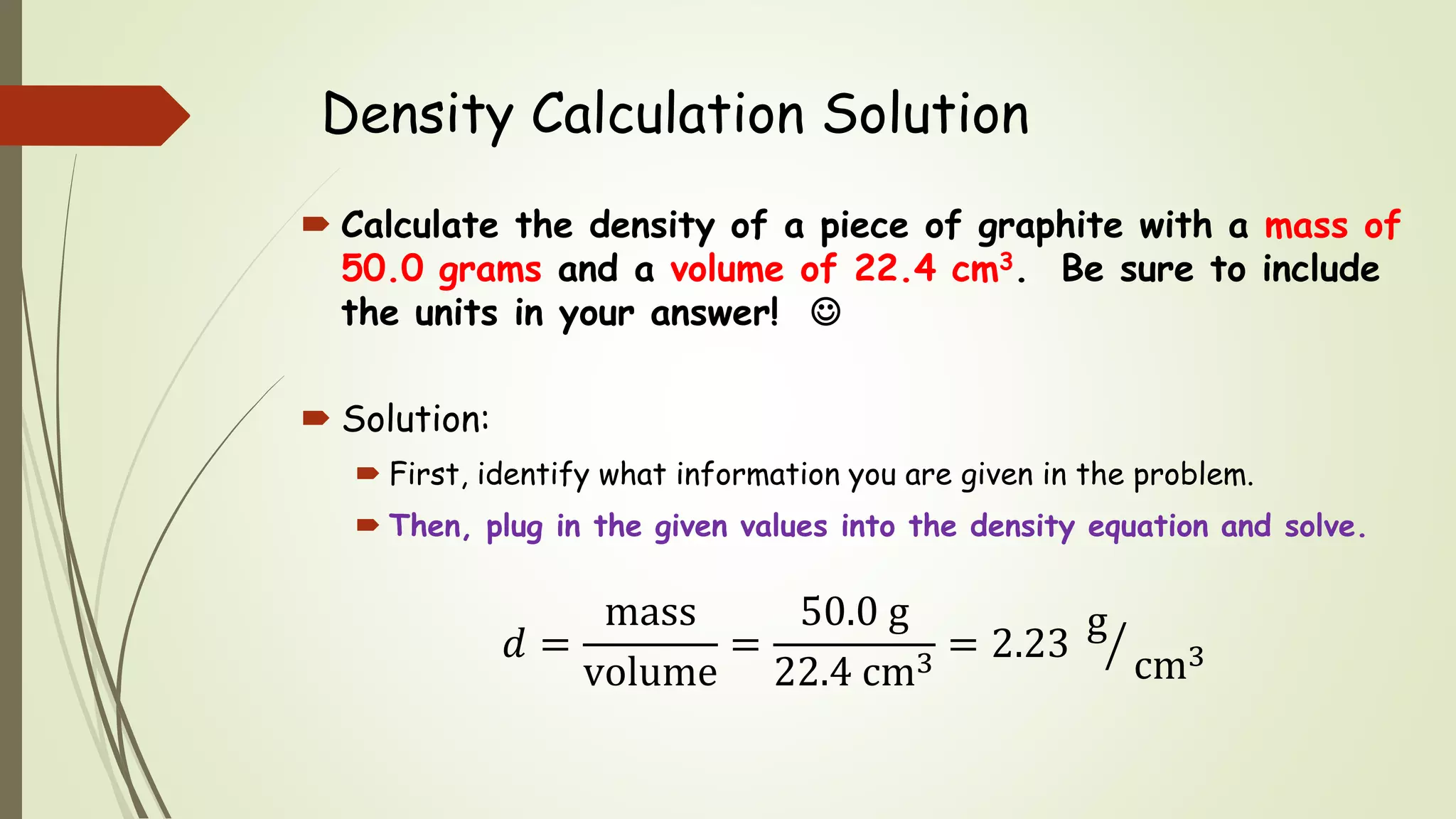
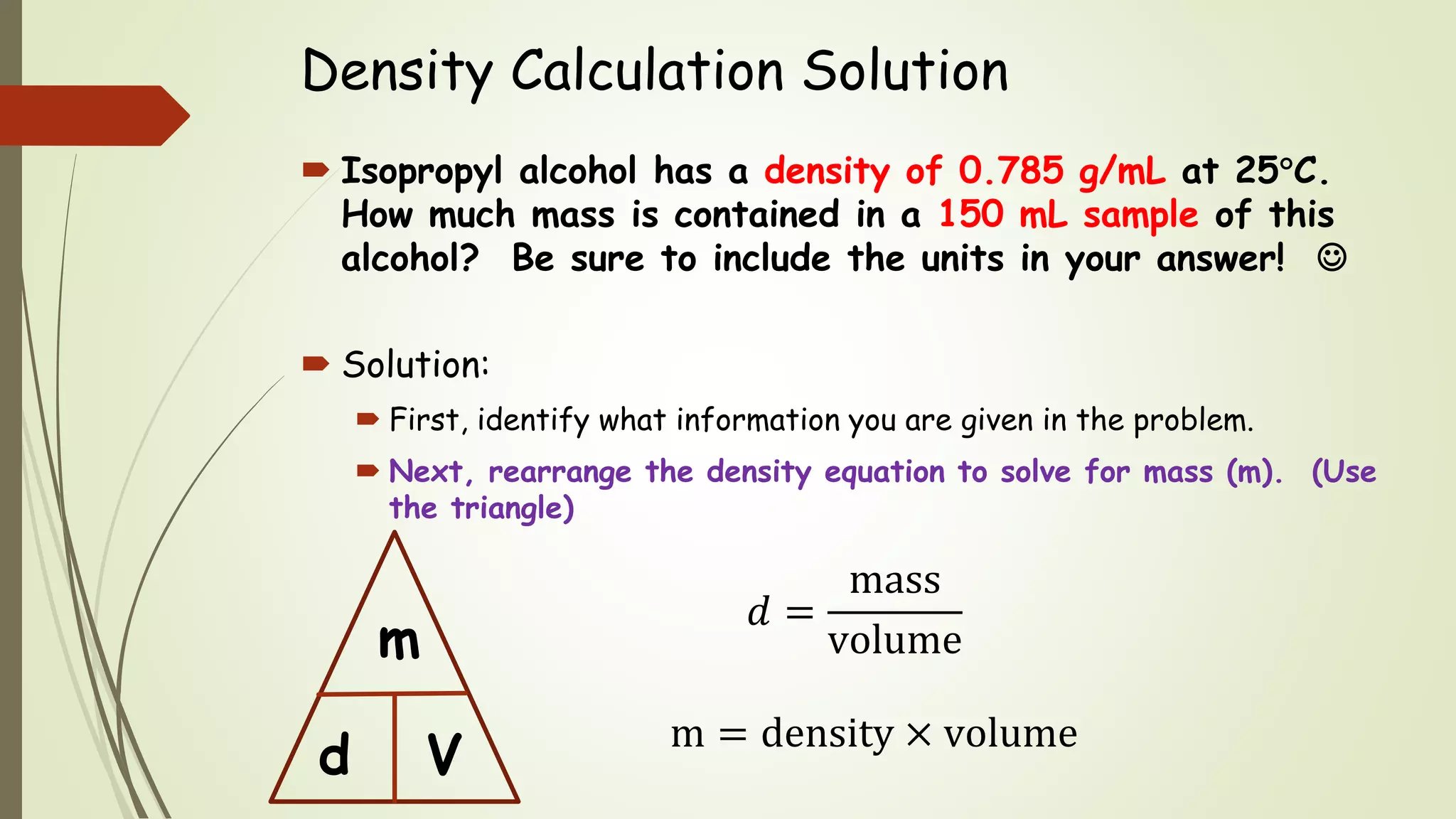
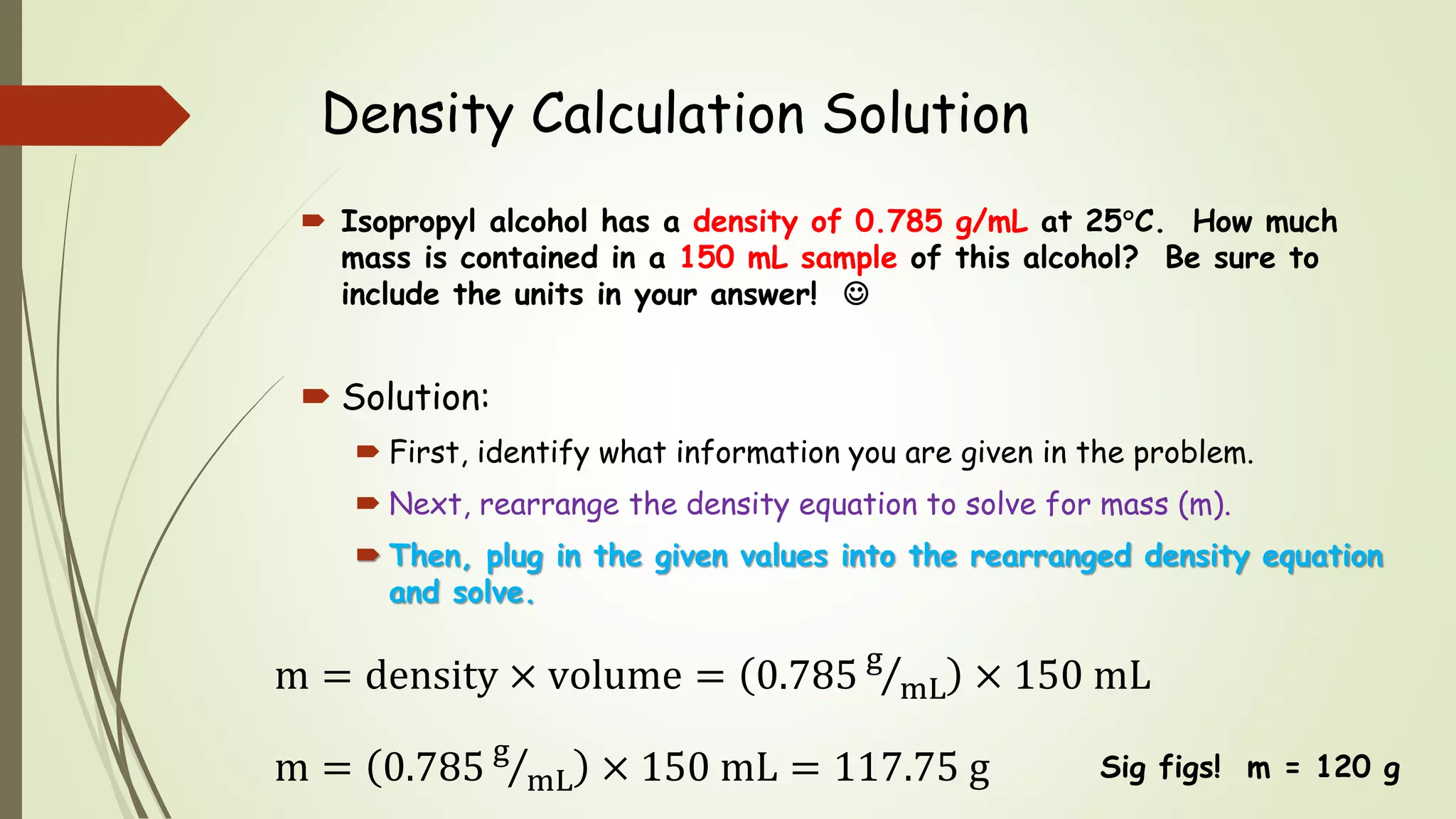
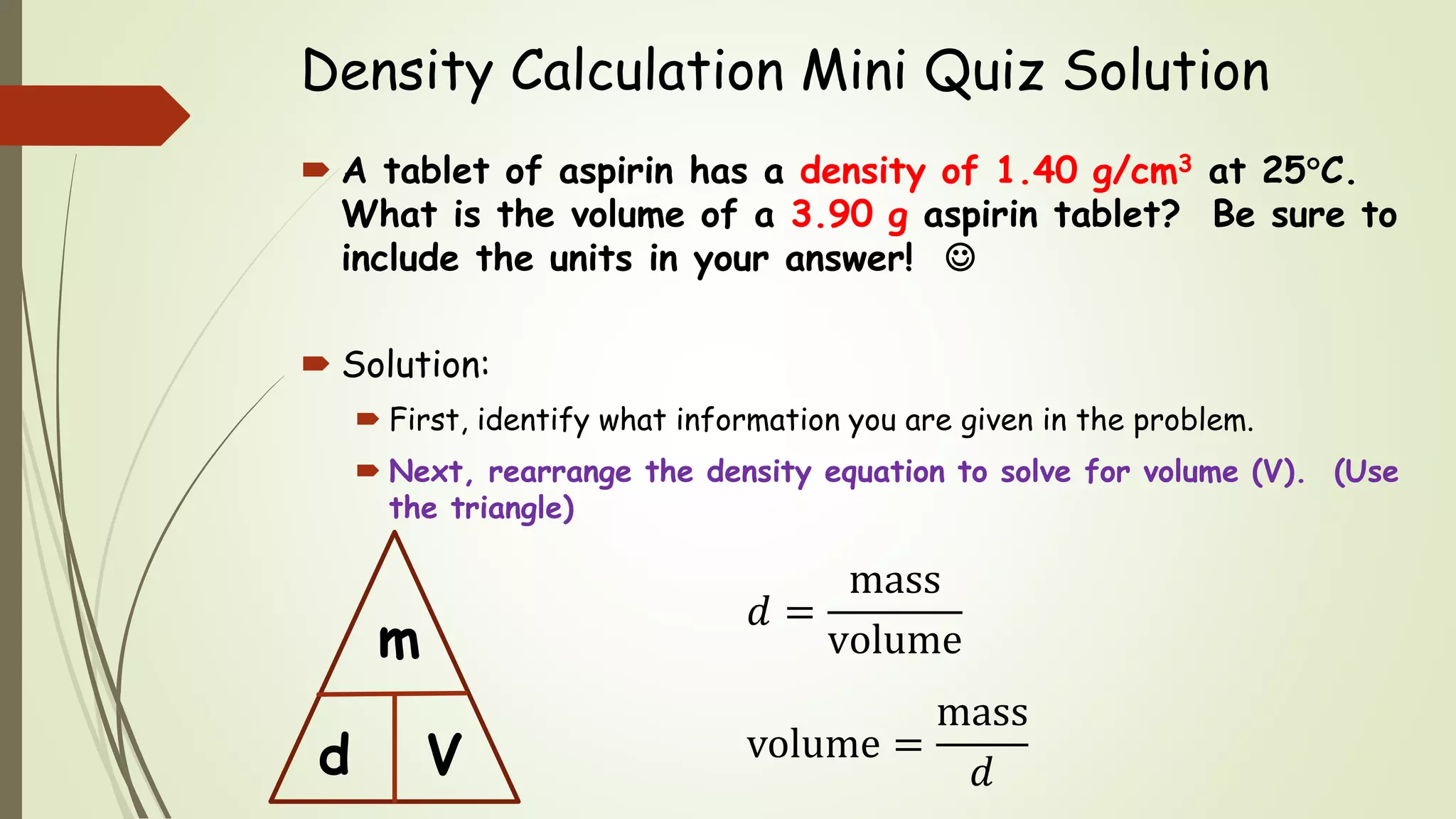
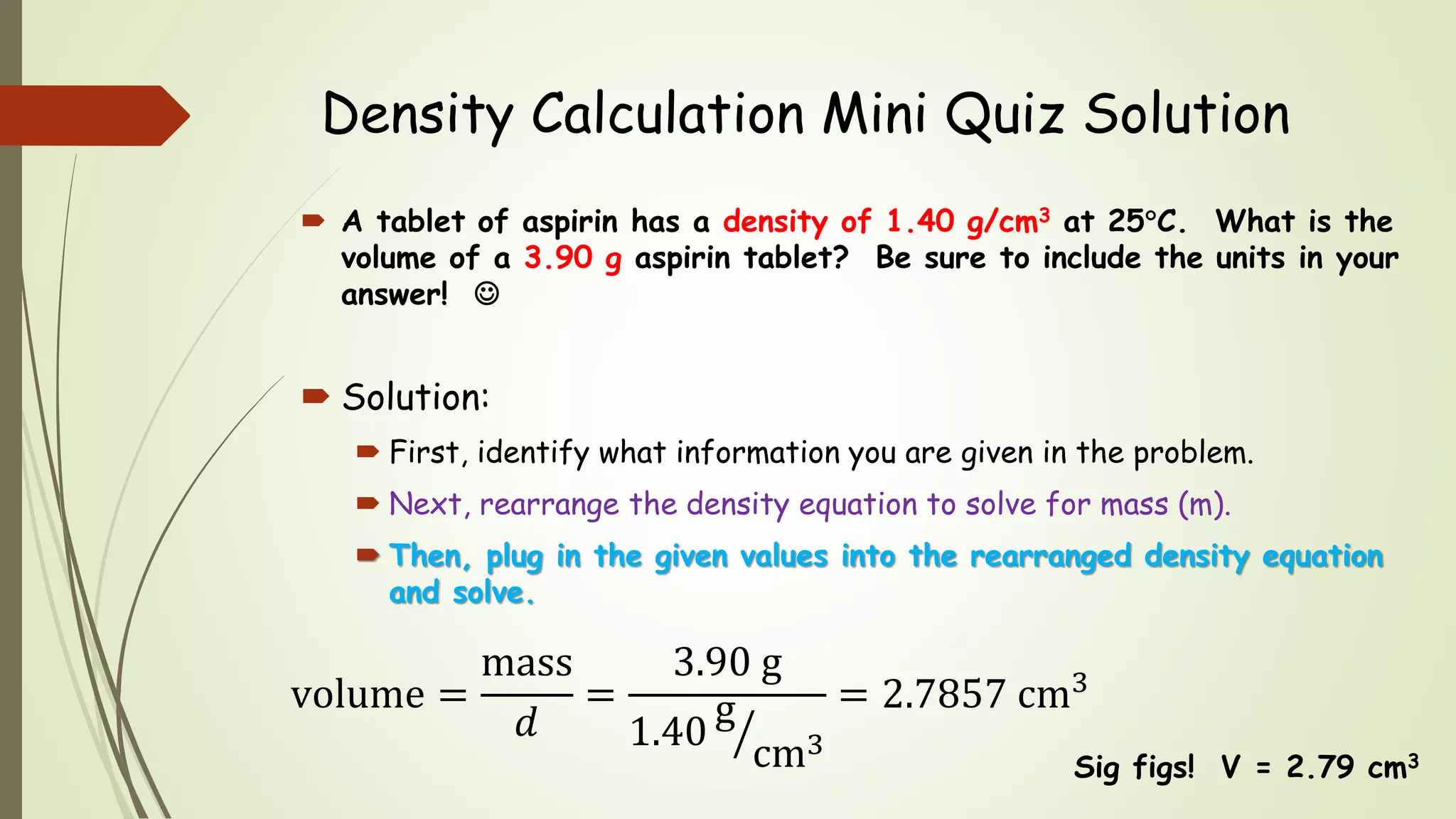
The document defines density and describes how to calculate it using the equation density = mass/volume. Density is a measure of how concentrated a substance is and has units of mass/volume (e.g. g/mL). Examples show how to use the density equation to calculate density, mass, or volume by rearranging the terms. Practice problems demonstrate calculating density, mass, and volume for different substances using the given values and density equation.