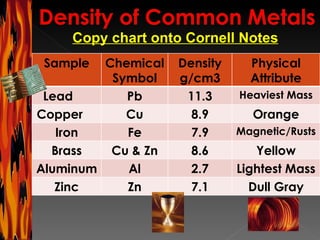
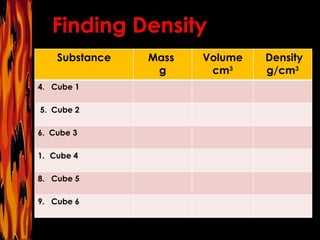
The document provides information about cooperative group roles and an introduction to density. It defines density as a ratio of mass to volume and explains that density remains the same even if the volume of a substance changes. It lists common materials and their densities and includes sample density problems and a chart to complete.