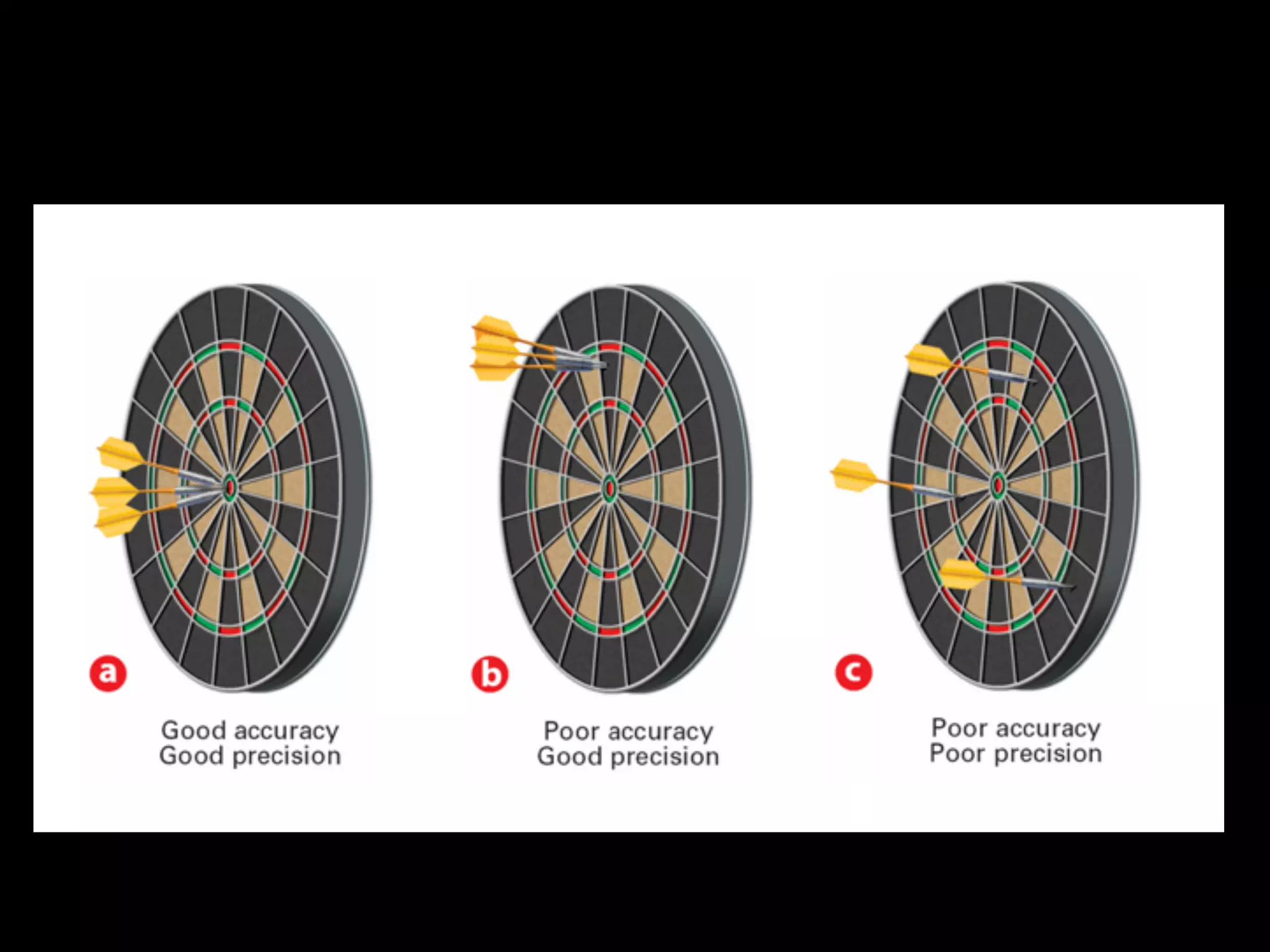
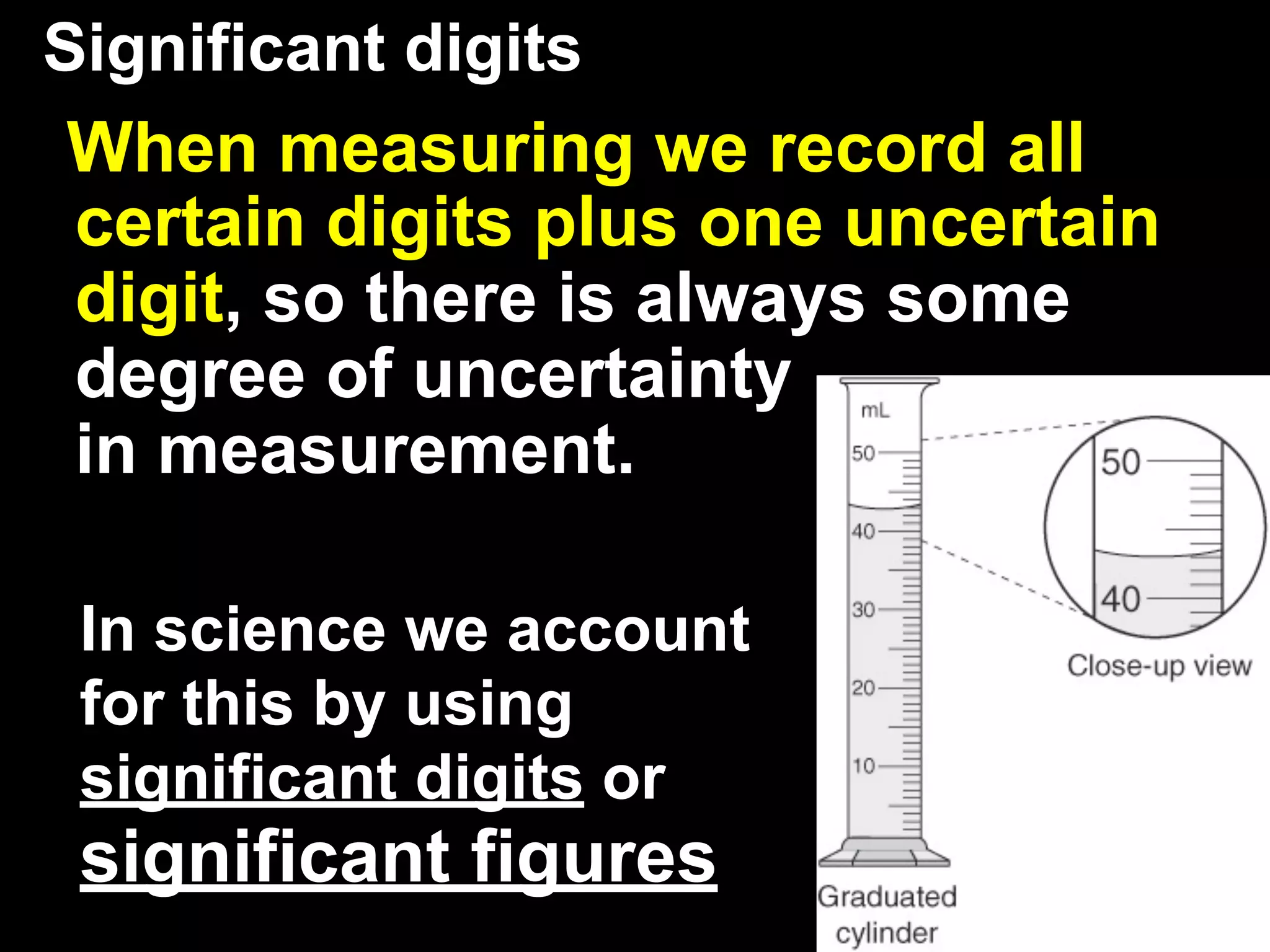
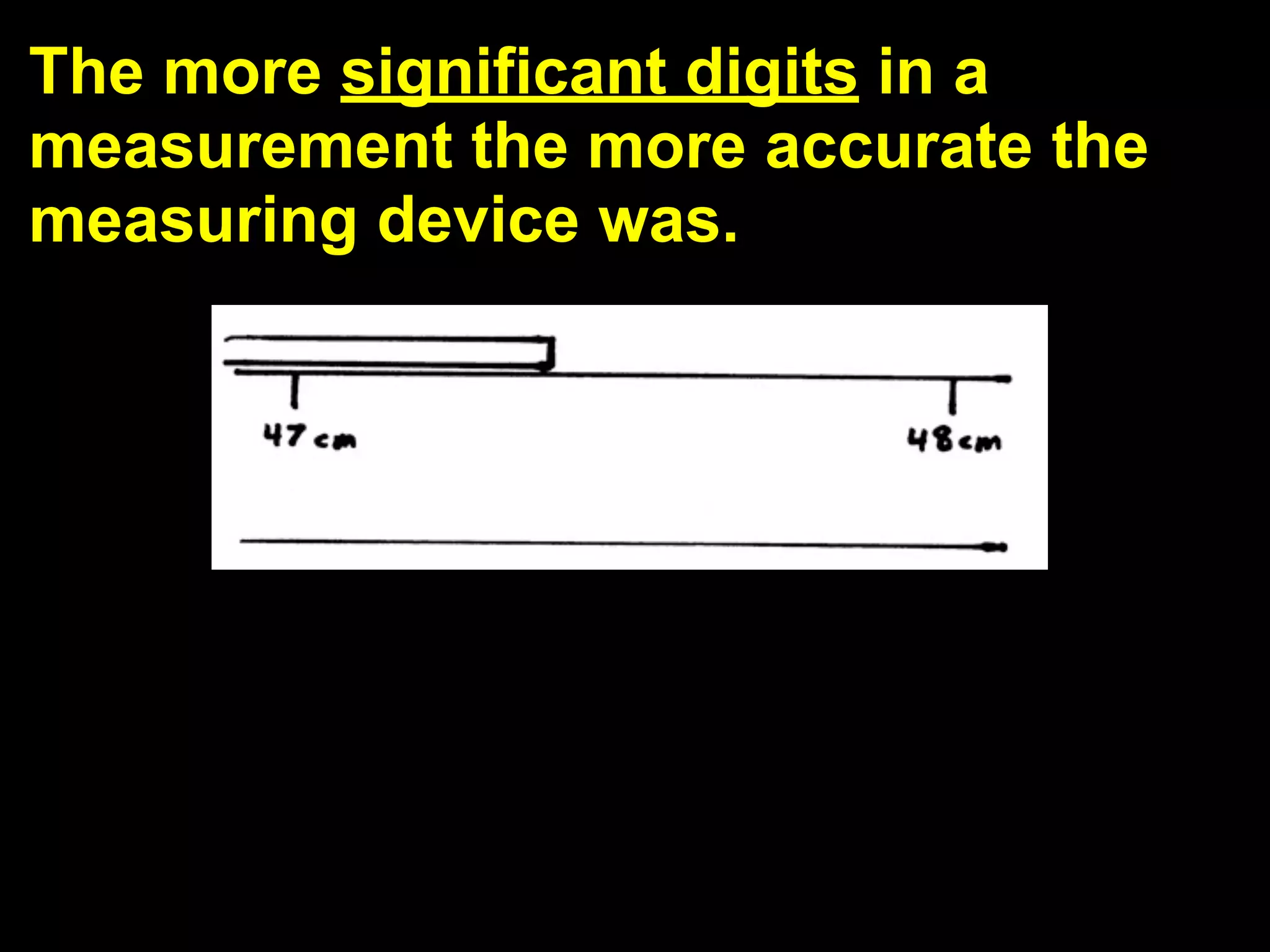
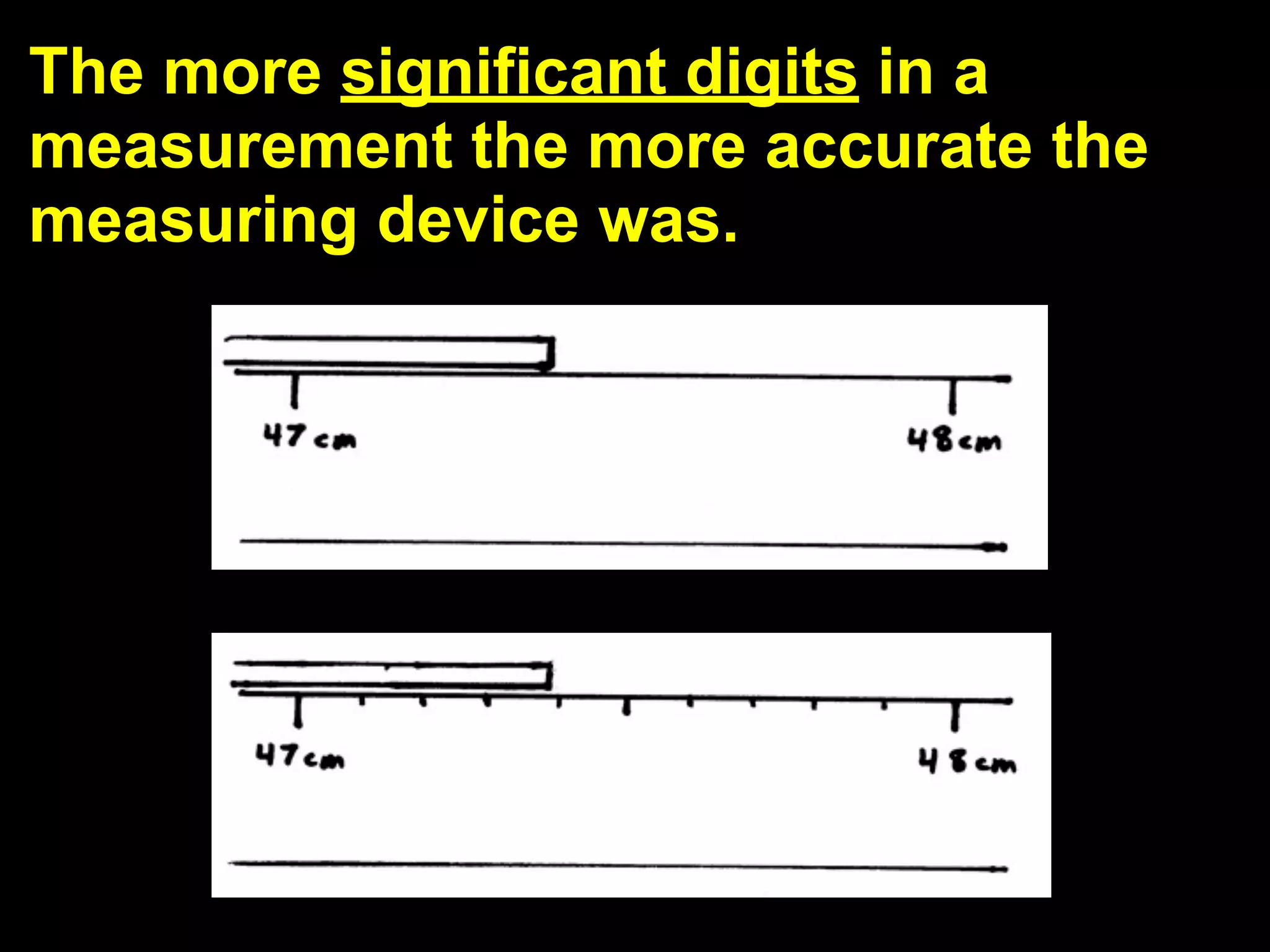
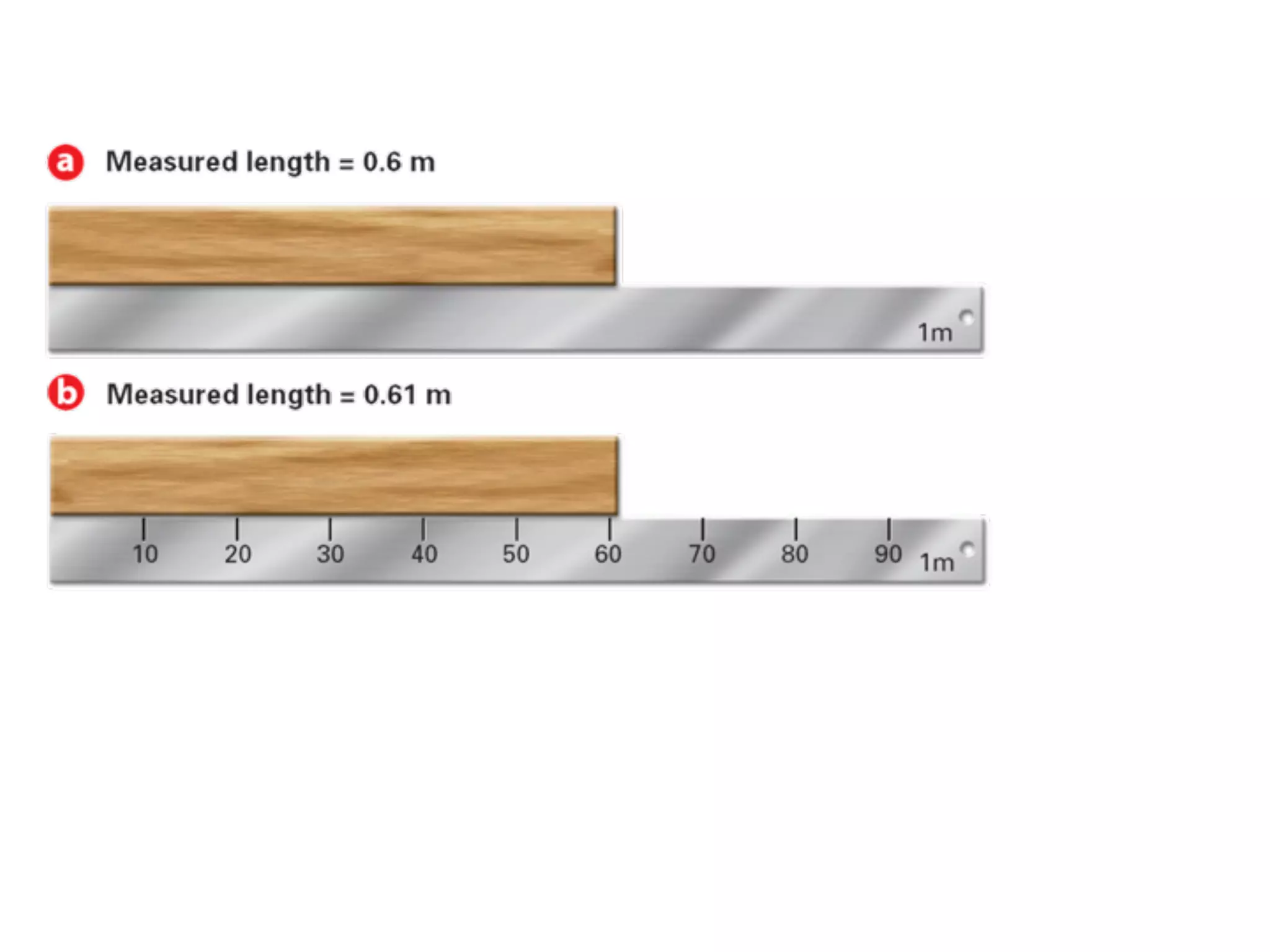
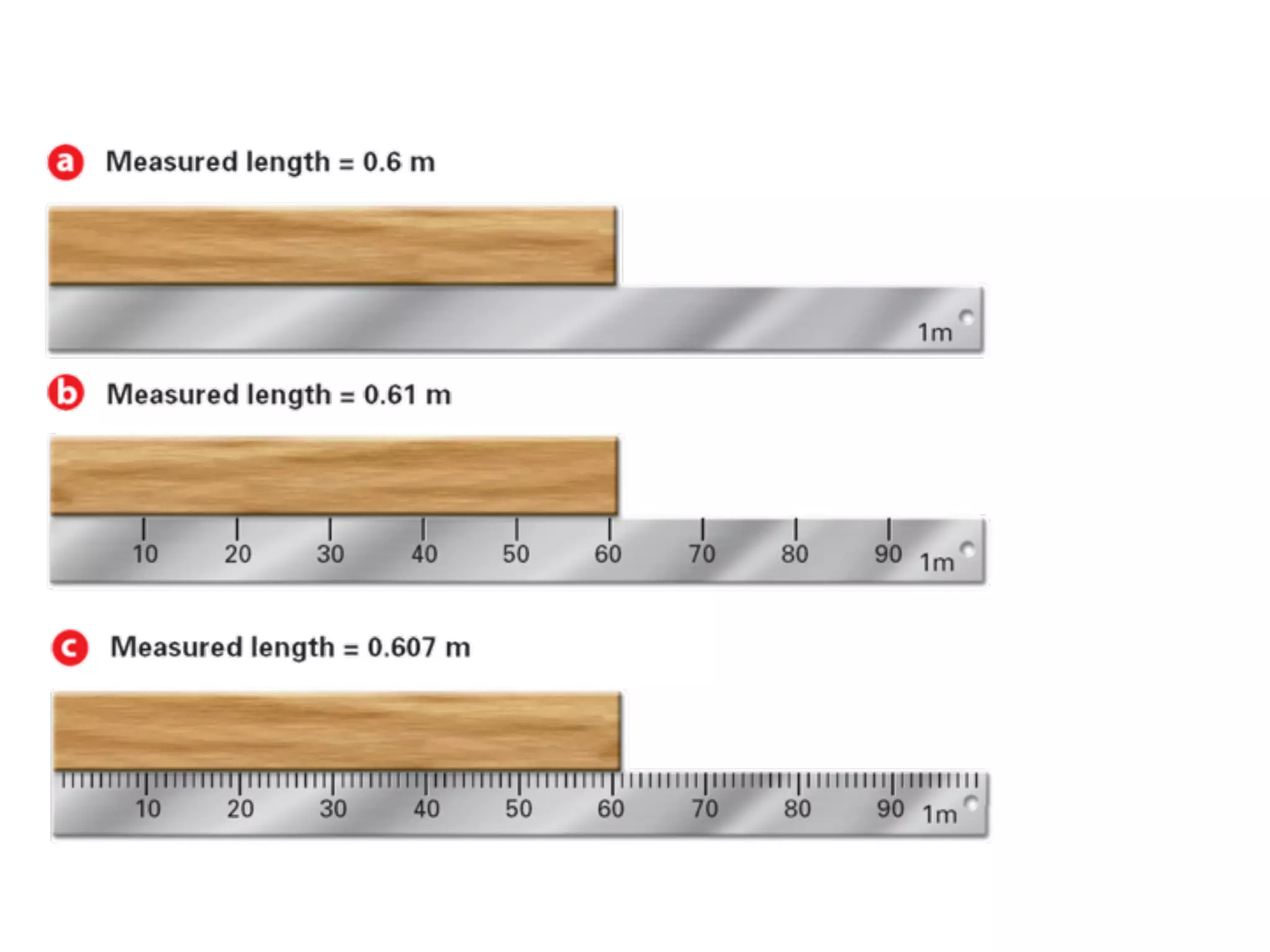
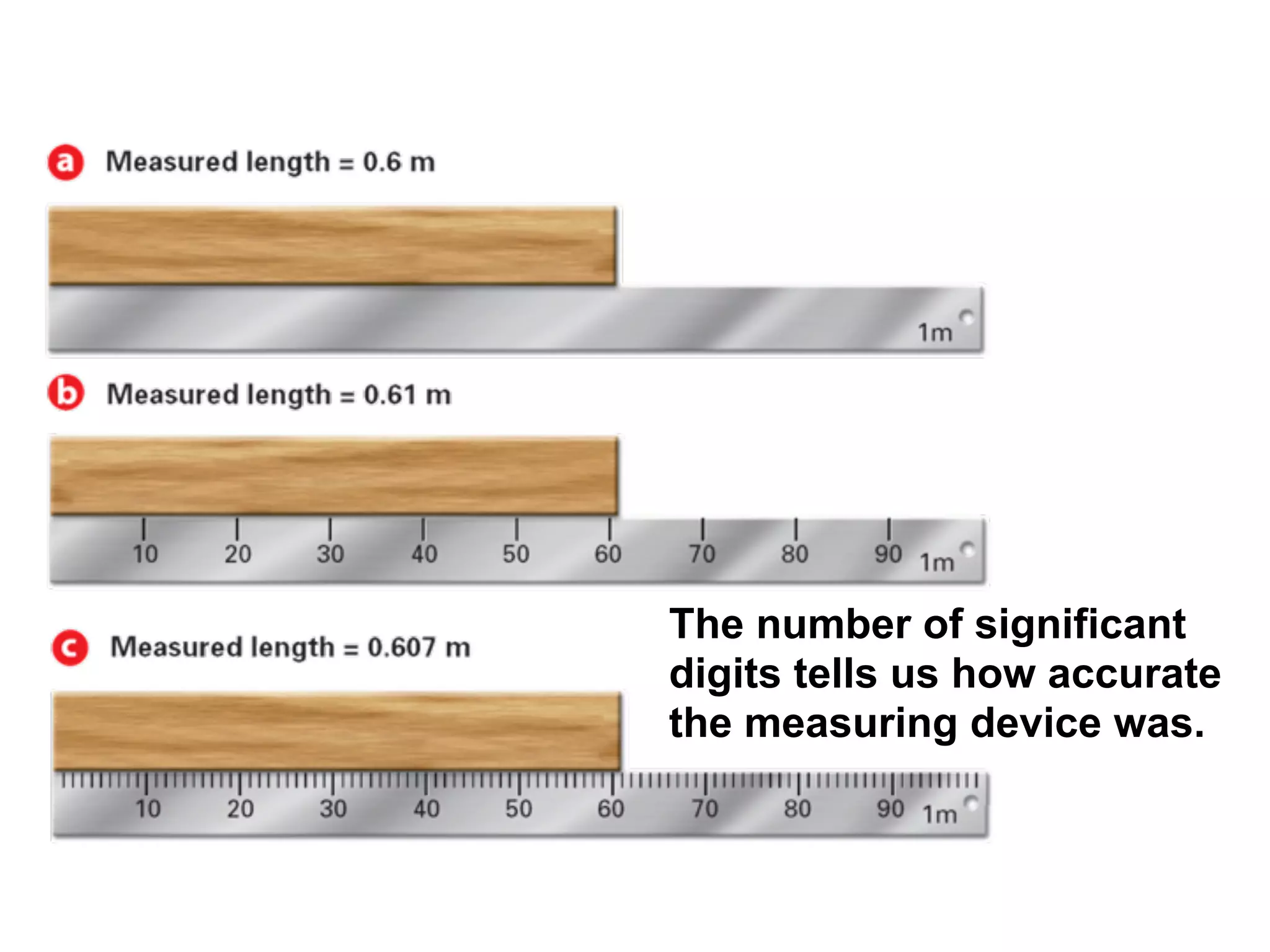
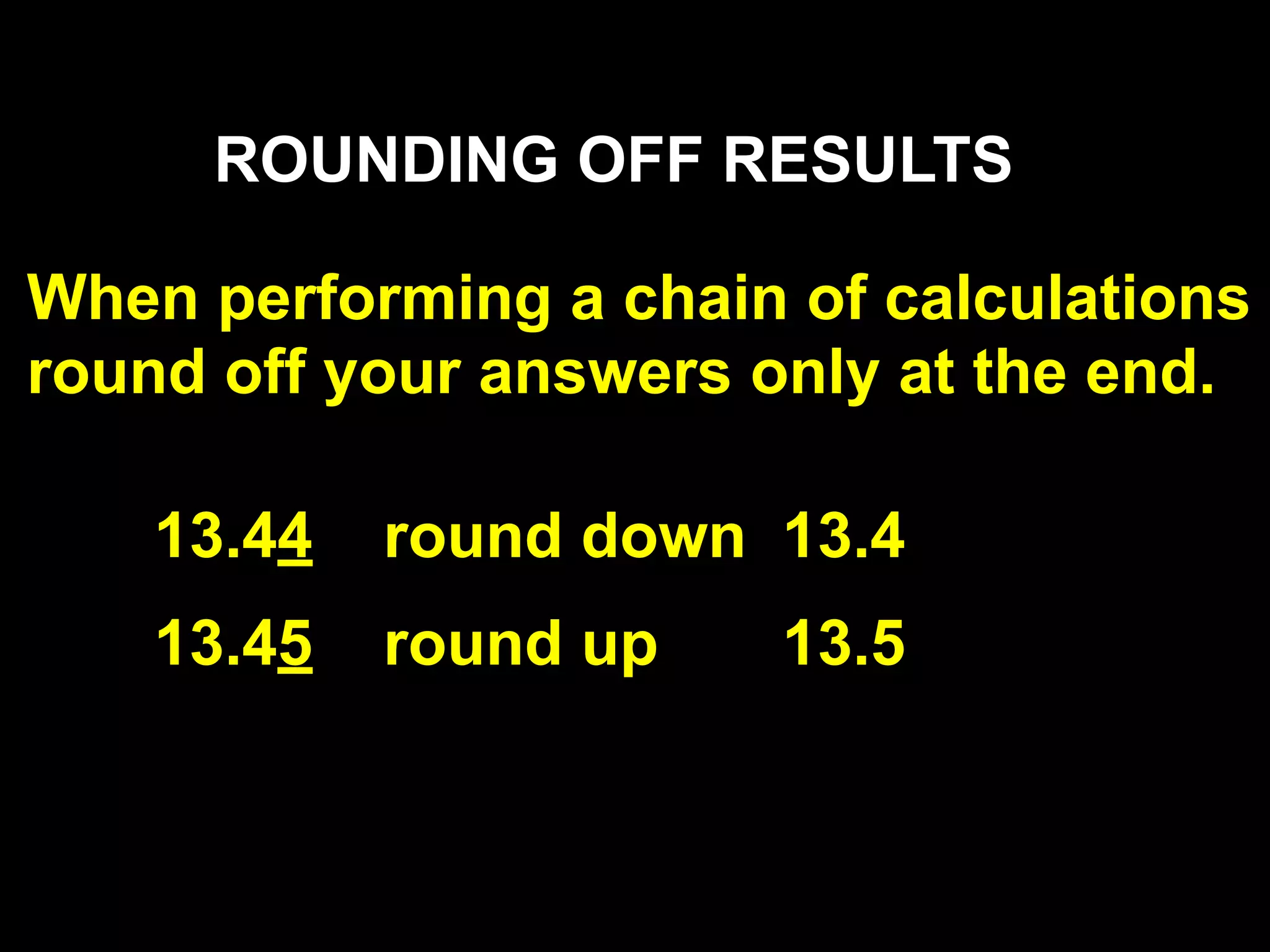
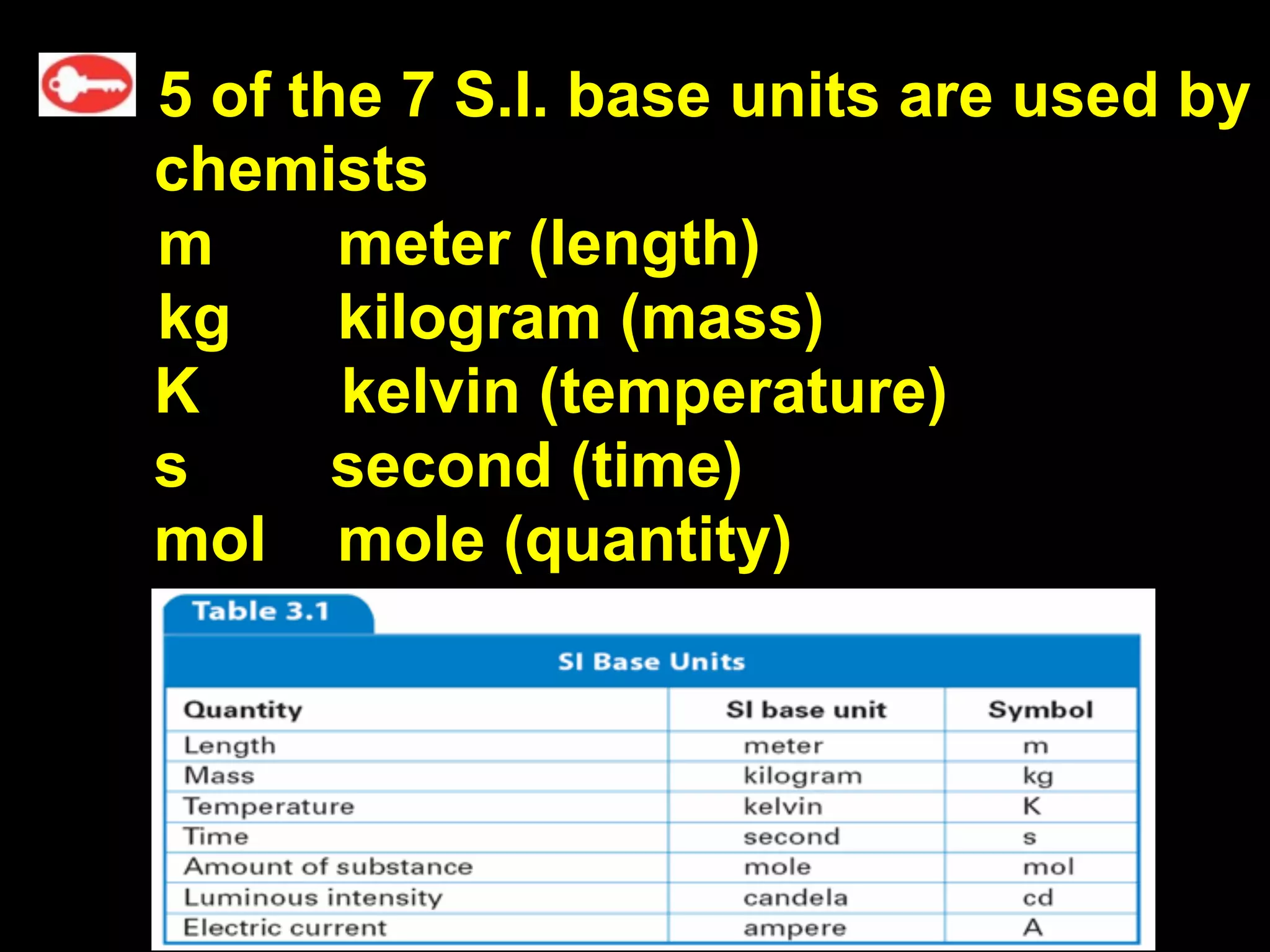
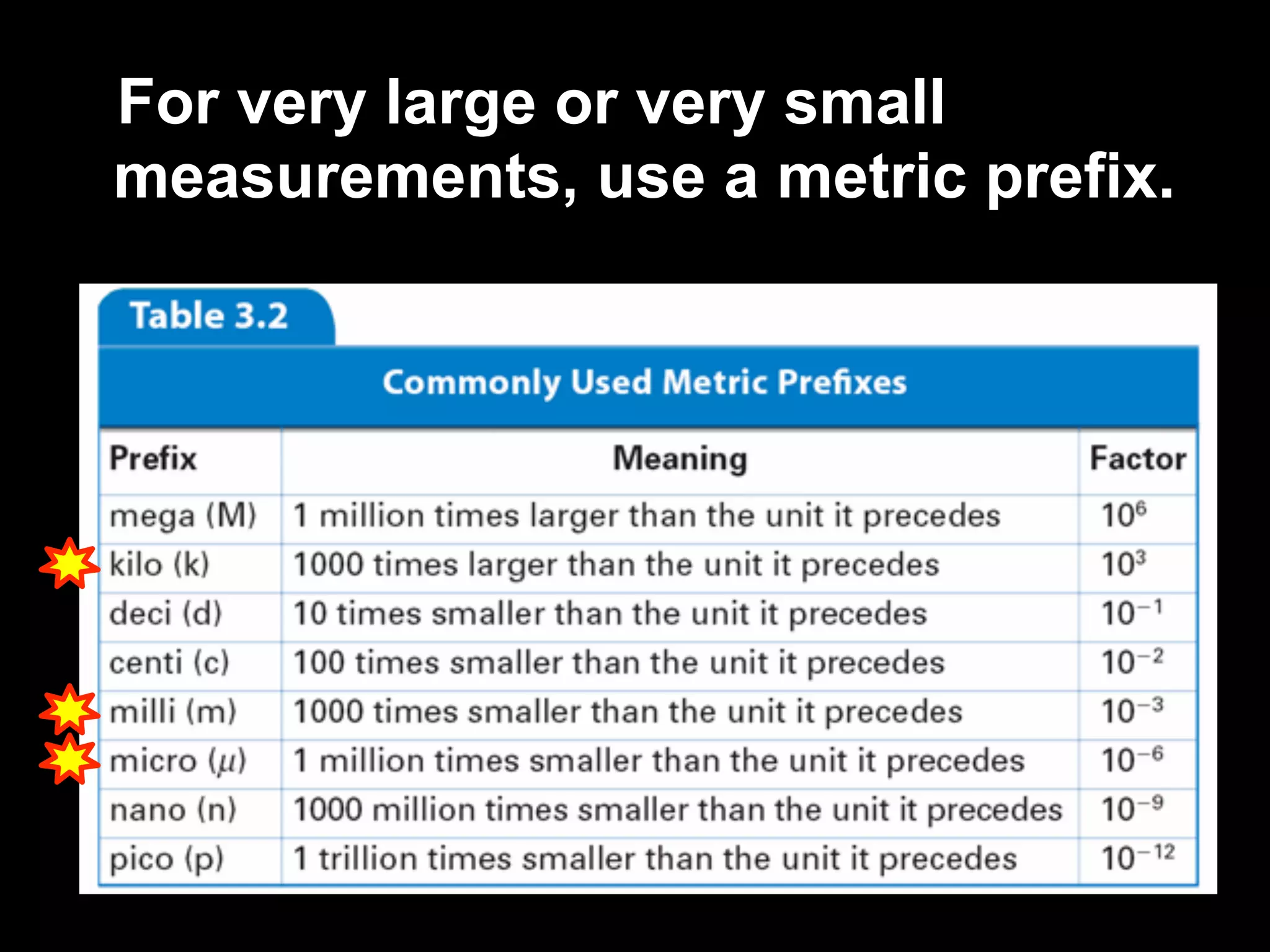
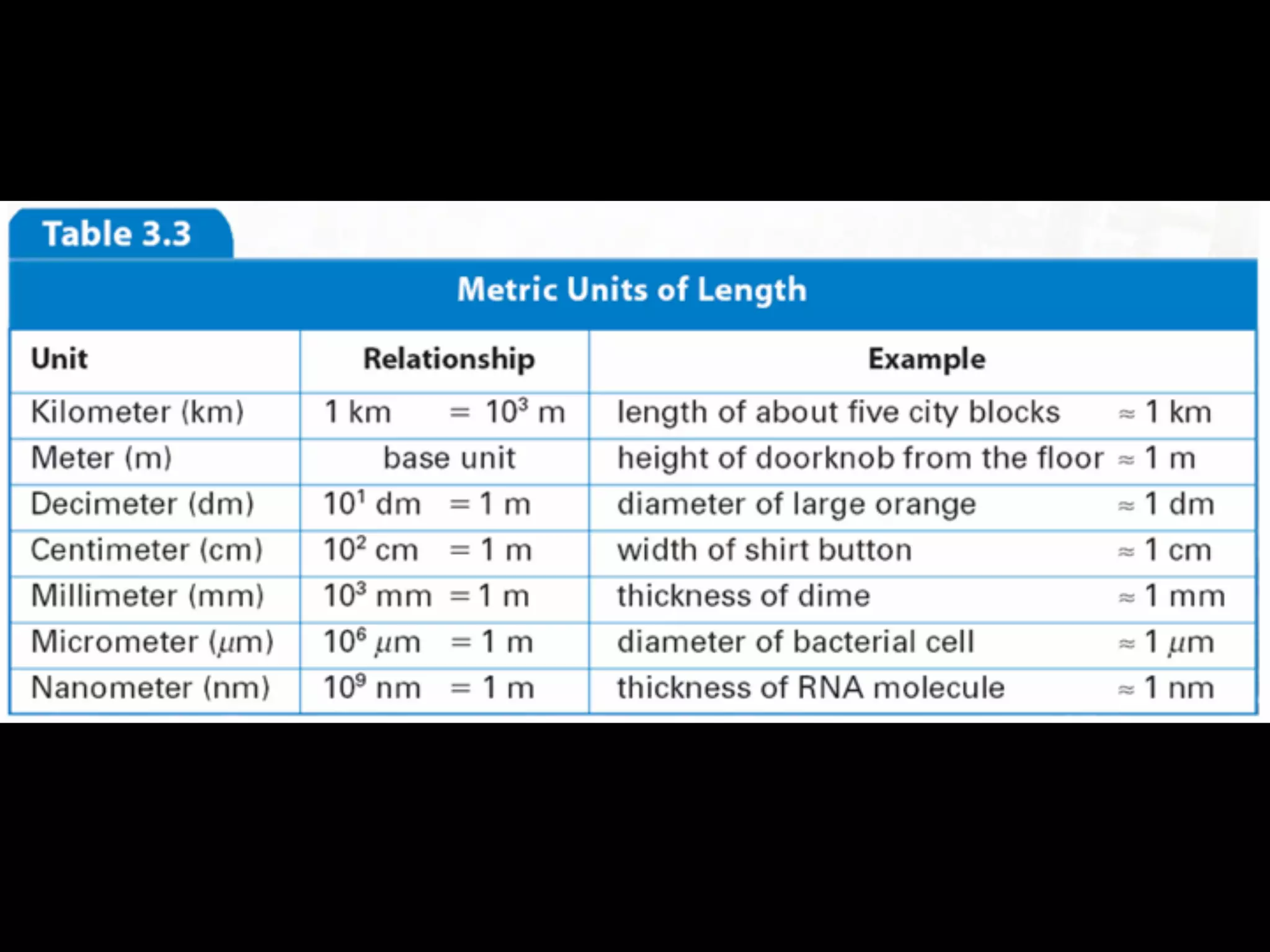
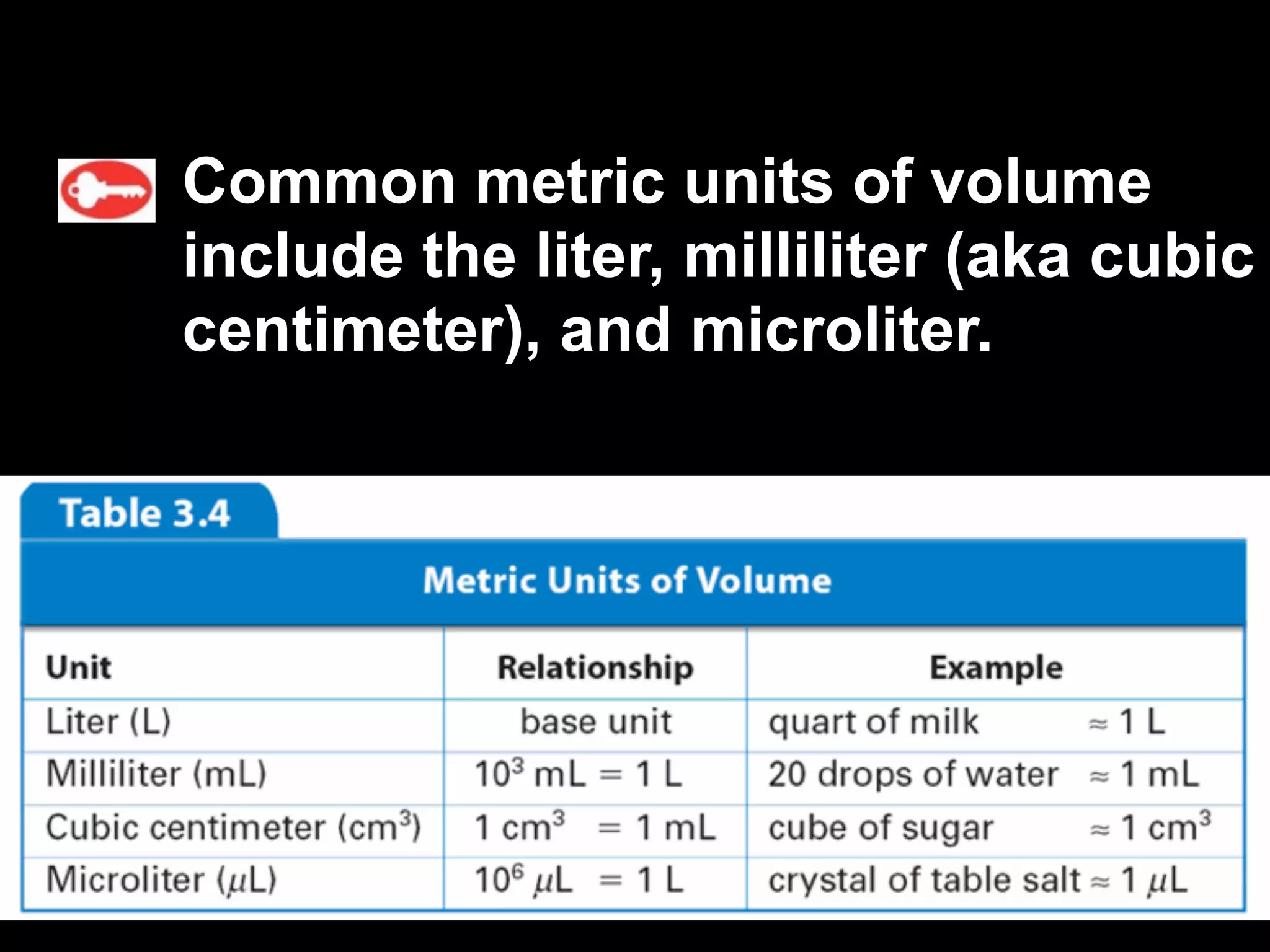
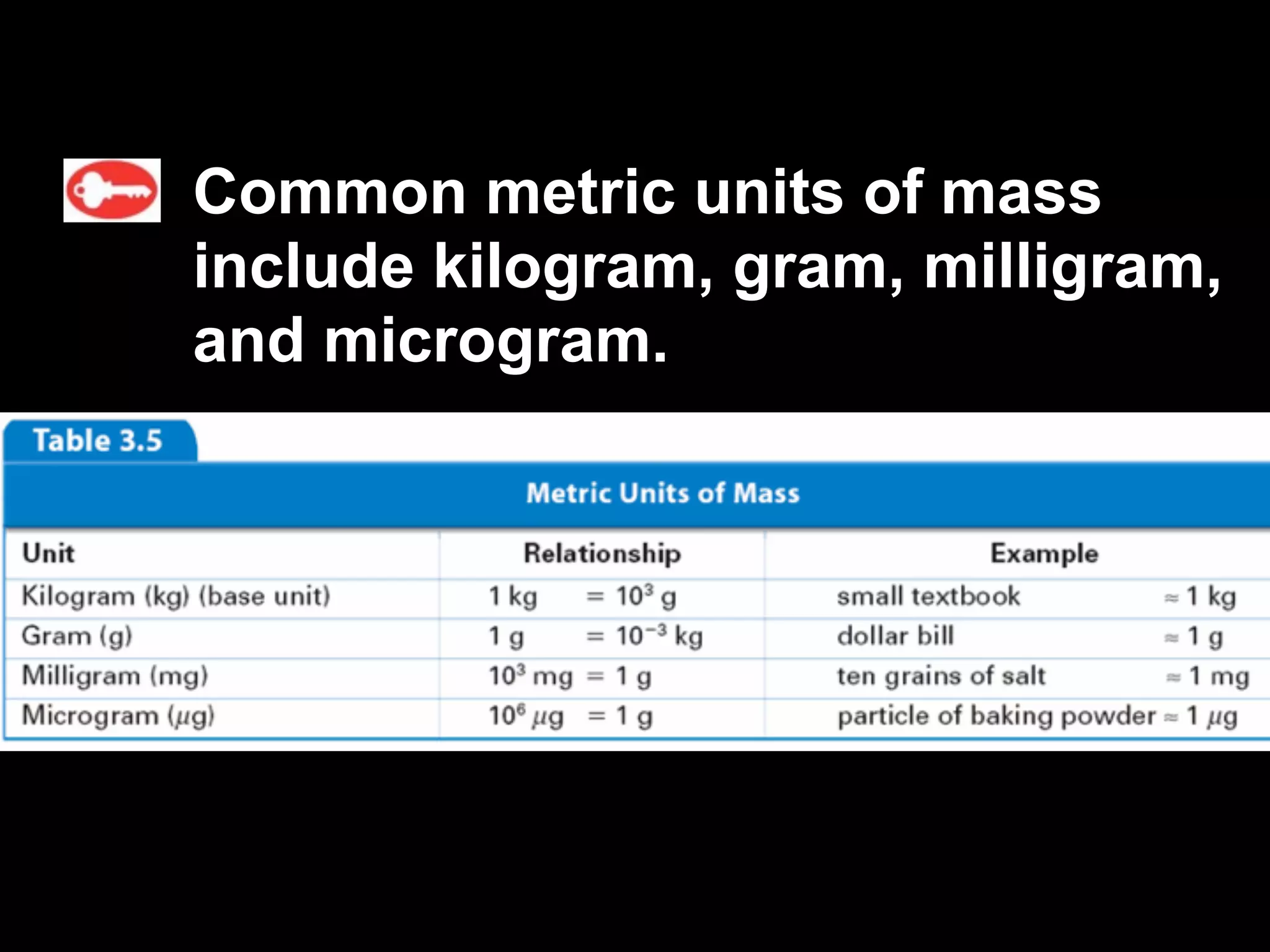
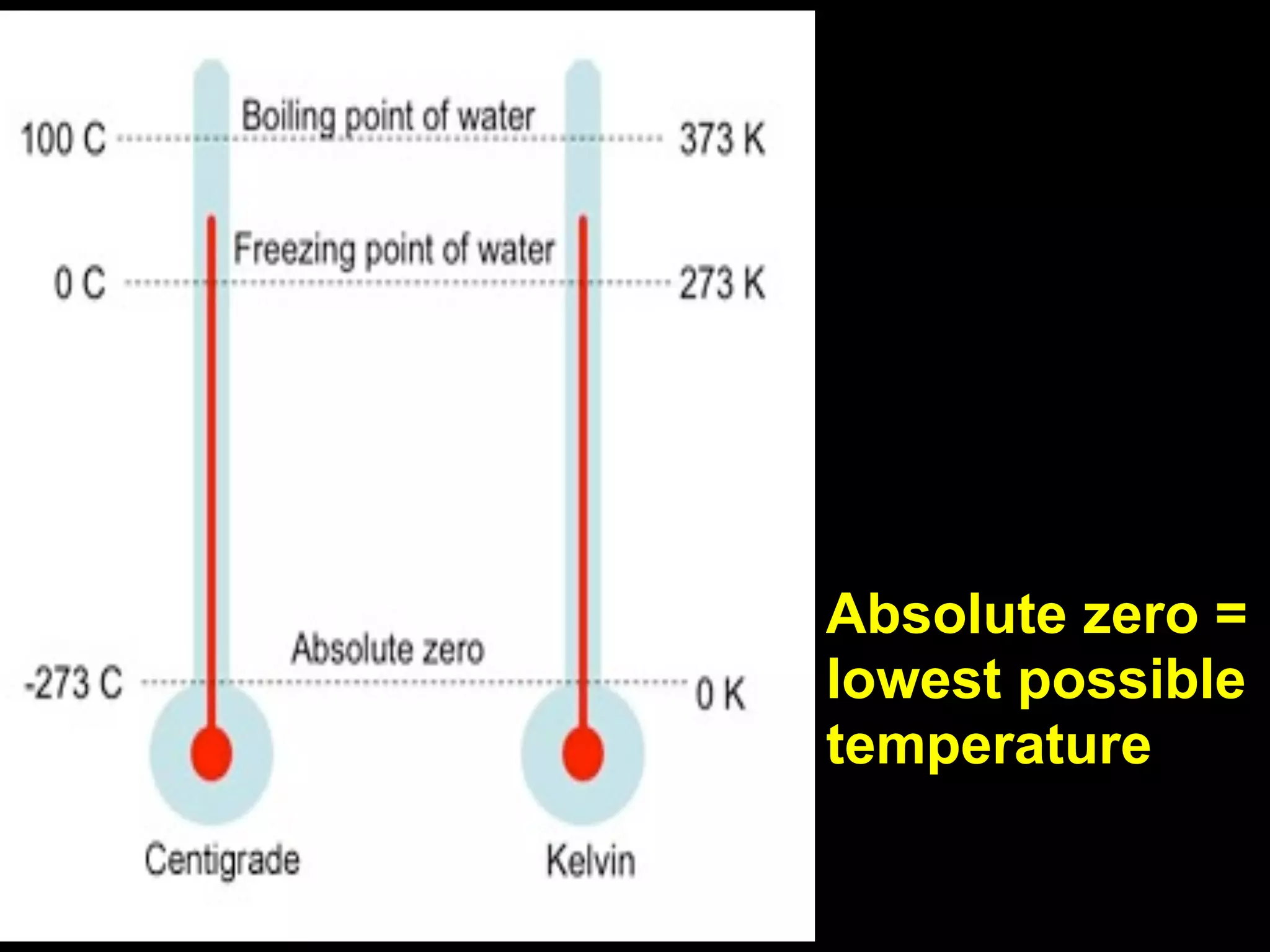
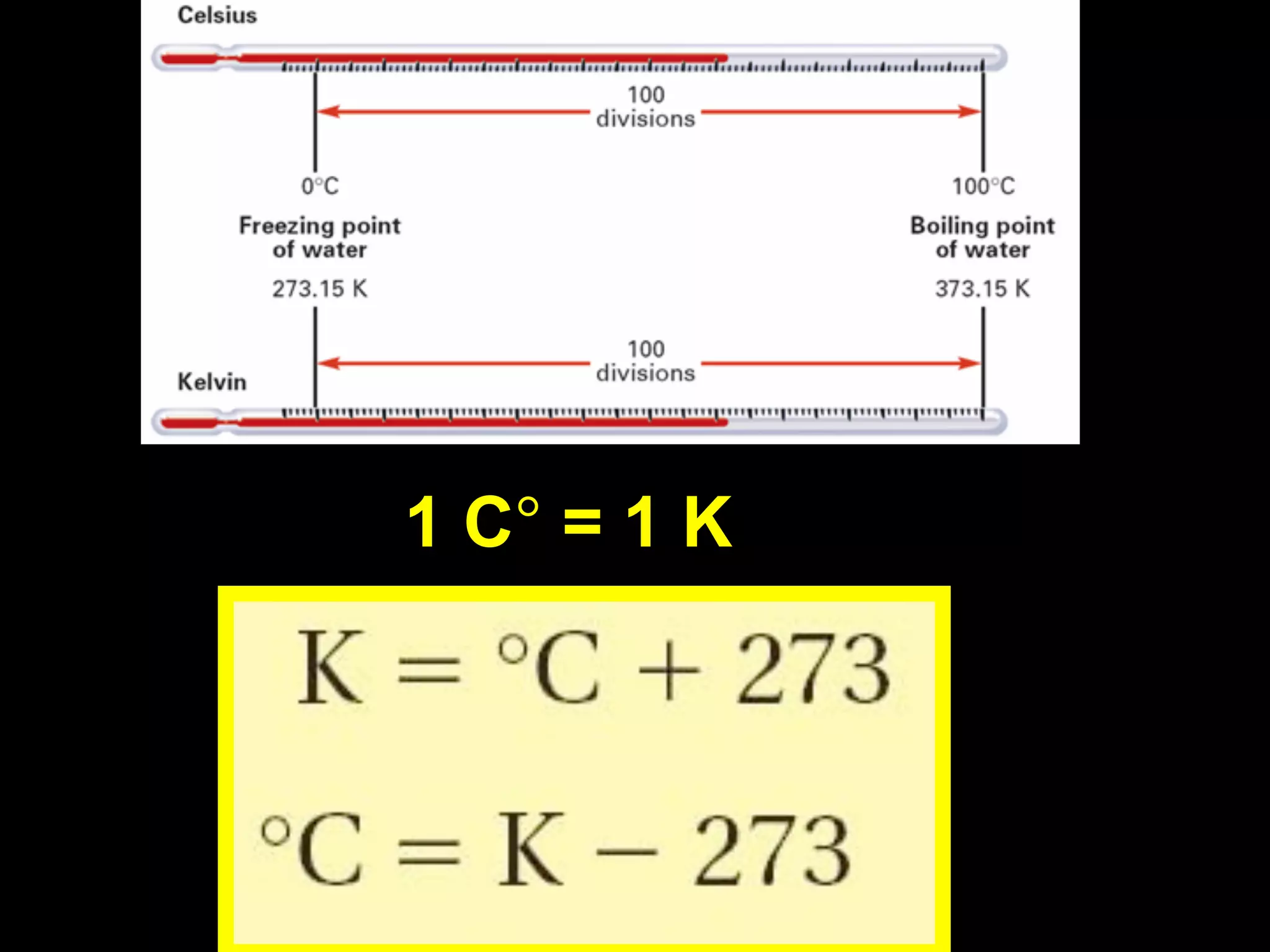
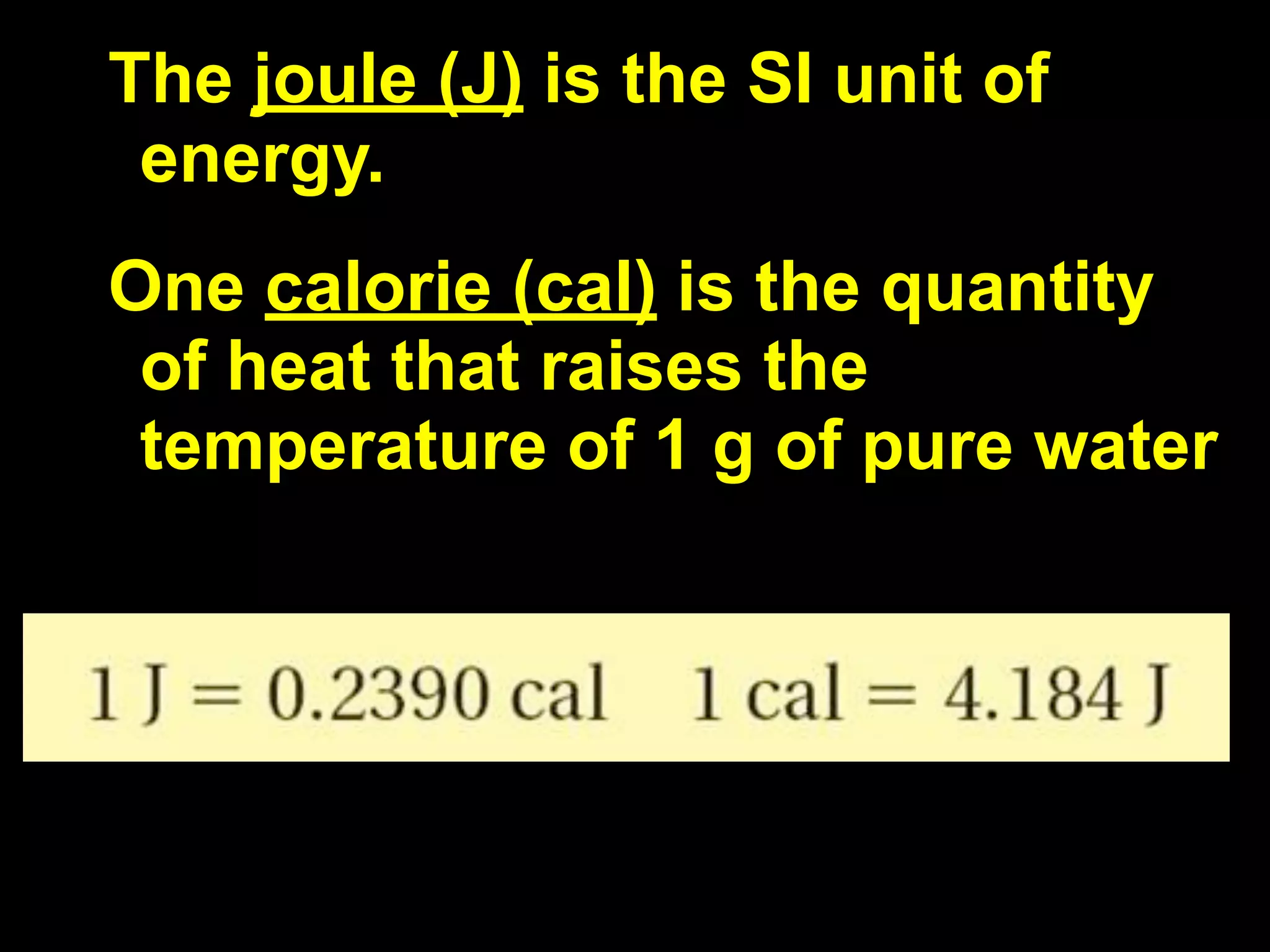
This document discusses scientific notation and units of measurement. It introduces scientific notation as a way to represent very large or small numbers between 1 and 10 with an exponent. Accuracy refers to how close a measurement is to the true value, while precision refers to the agreement between repeated measurements. Significant figures indicate the precision or number of digits known in a measurement. Calculations are only as precise as the least precise input measurement. Proper rounding of results is also covered. Standard International (SI) units for length, mass, temperature, time, and amount are defined. Common metric units for volume, mass, and temperature are also discussed.