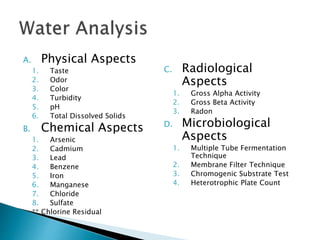
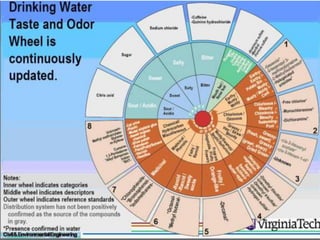
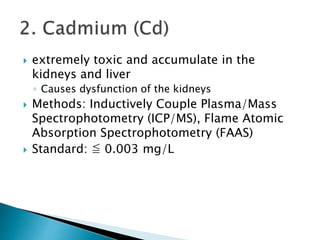
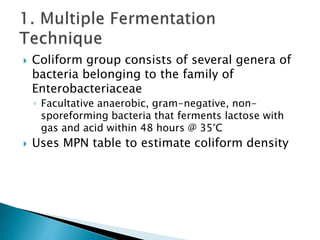
The document outlines various aspects of water quality assessment, encompassing physical, chemical, radiological, and microbiological parameters. It details methods for measuring these parameters, standards for acceptable levels, and health implications of various contaminants. Emphasis is placed on the importance of accurate data for informed decision-making and strict adherence to quality control procedures in laboratory analysis.