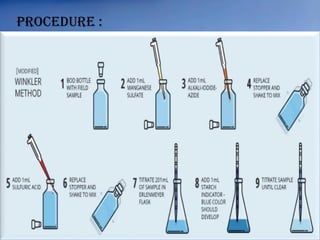
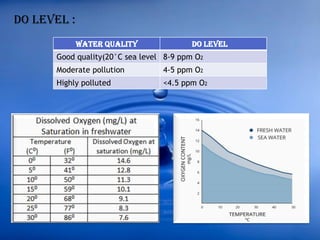
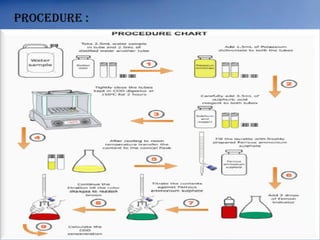
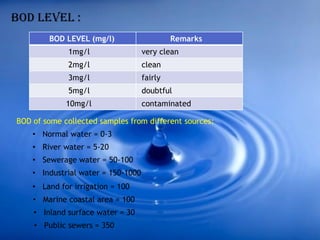
The document discusses dissolved oxygen (DO), chemical oxygen demand (COD), and biological oxygen demand (BOD) as key indicators of water quality and pollution levels. It details methods for measuring these parameters, including the Winkler method for DO and the use of potassium dichromate for COD. Additionally, it outlines the significance of BOD in assessing organic pollution in water and provides standard values for evaluating water quality.