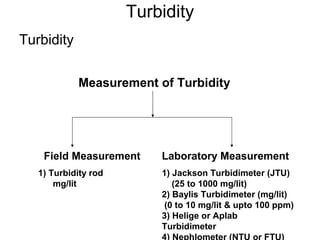
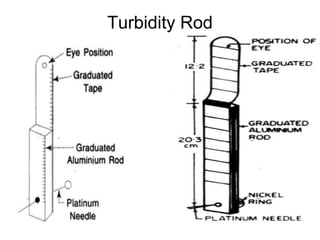
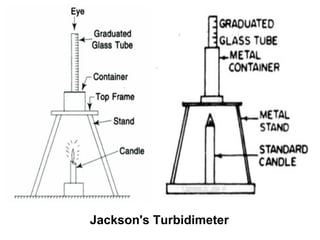
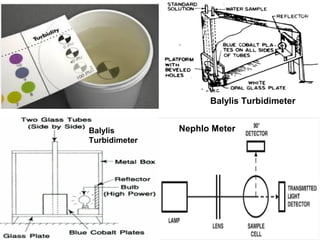
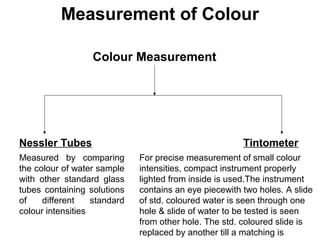
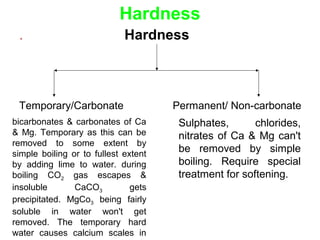
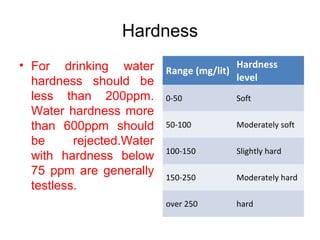
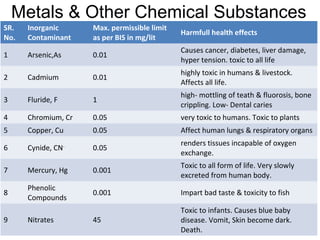
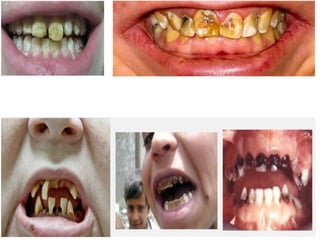
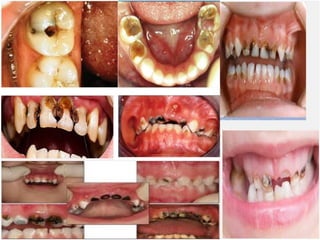
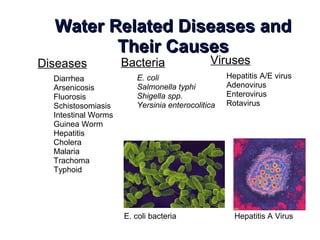
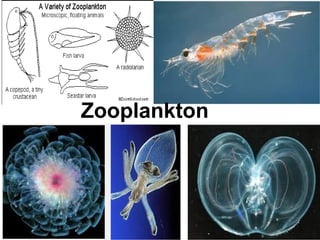
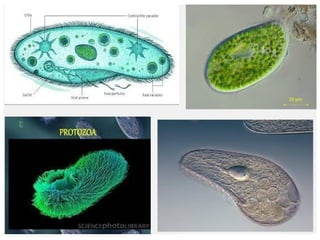
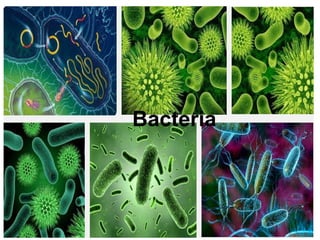
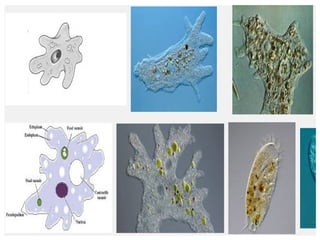
The document discusses the quality of water, emphasizing its physical, chemical, and biological characteristics, along with the standards for safe drinking water. It highlights the global water crisis, various contaminants, their health implications, and the necessary methods for measuring water quality attributes such as turbidity, color, taste, odor, temperature, and conductivity. Additionally, it covers the importance of analyzing water to determine its purity, classify sources, and outline purification processes.