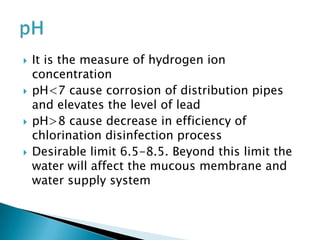
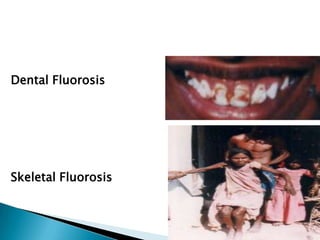
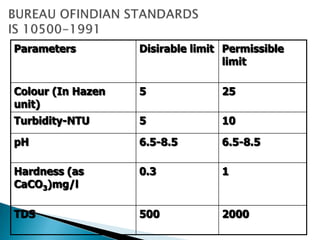
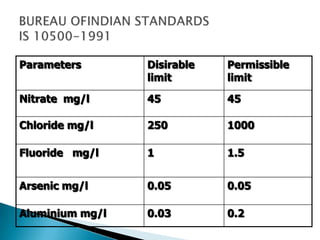
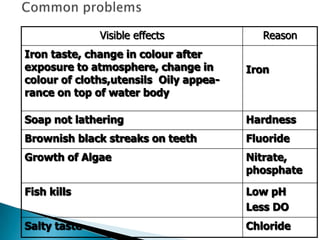
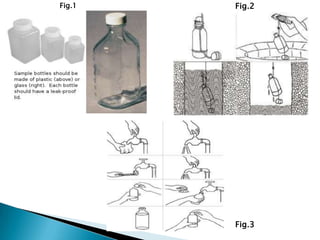
This document discusses water quality parameters and testing. It outlines that water quality depends on physical, chemical and biological characteristics. Approximately 25% of the world's population lacks access to potable water. Key water quality parameters that are tested include turbidity, pH, hardness, nitrates, chlorides, fluoride, and heavy metals. Testing methods examine parameters like color, odor, dissolved solids, and presence of bacteria, protozoa, and other microorganisms. Maintaining high-quality drinking water requires regular monitoring, sample collection, and analysis of water sources.