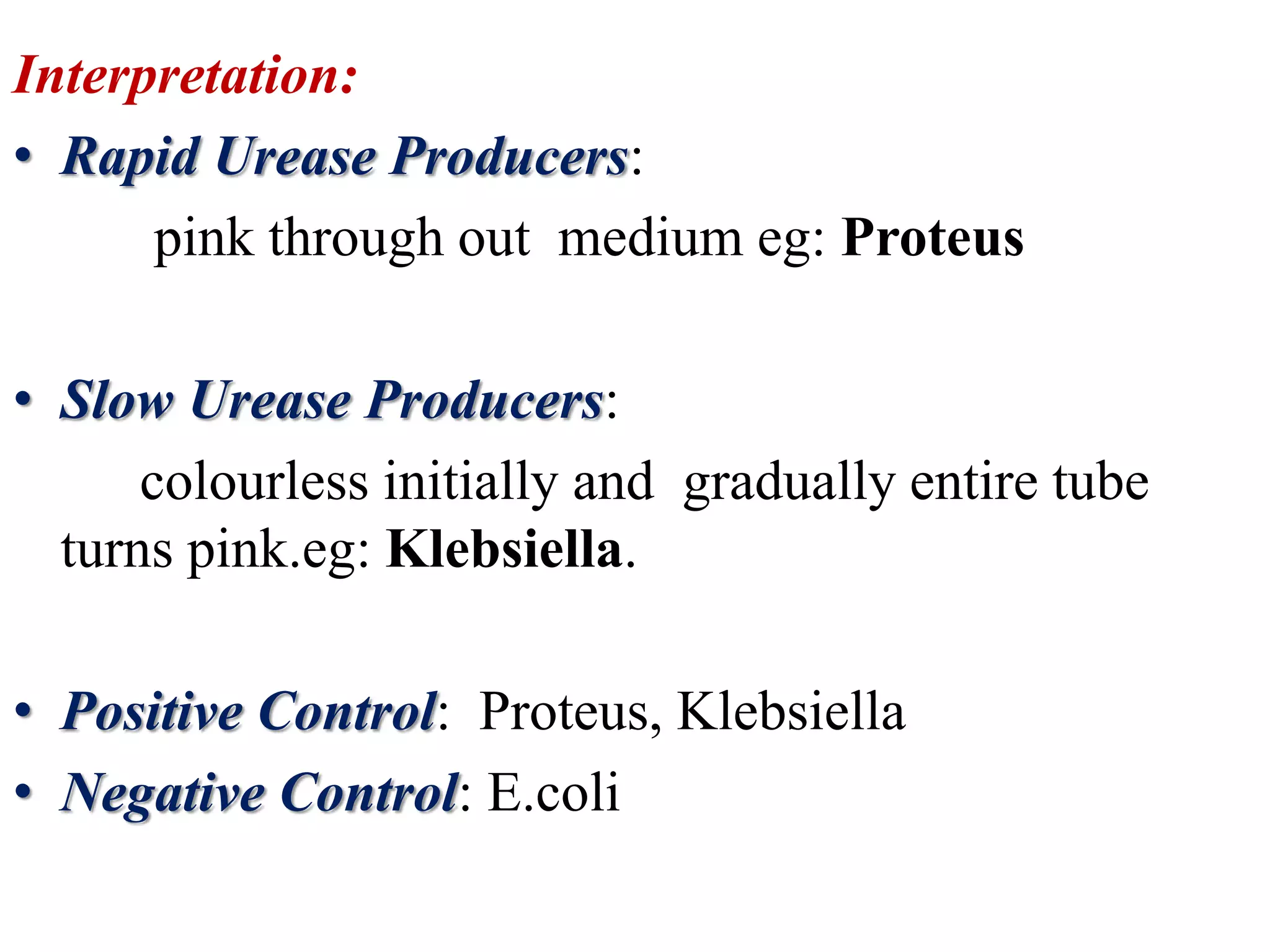
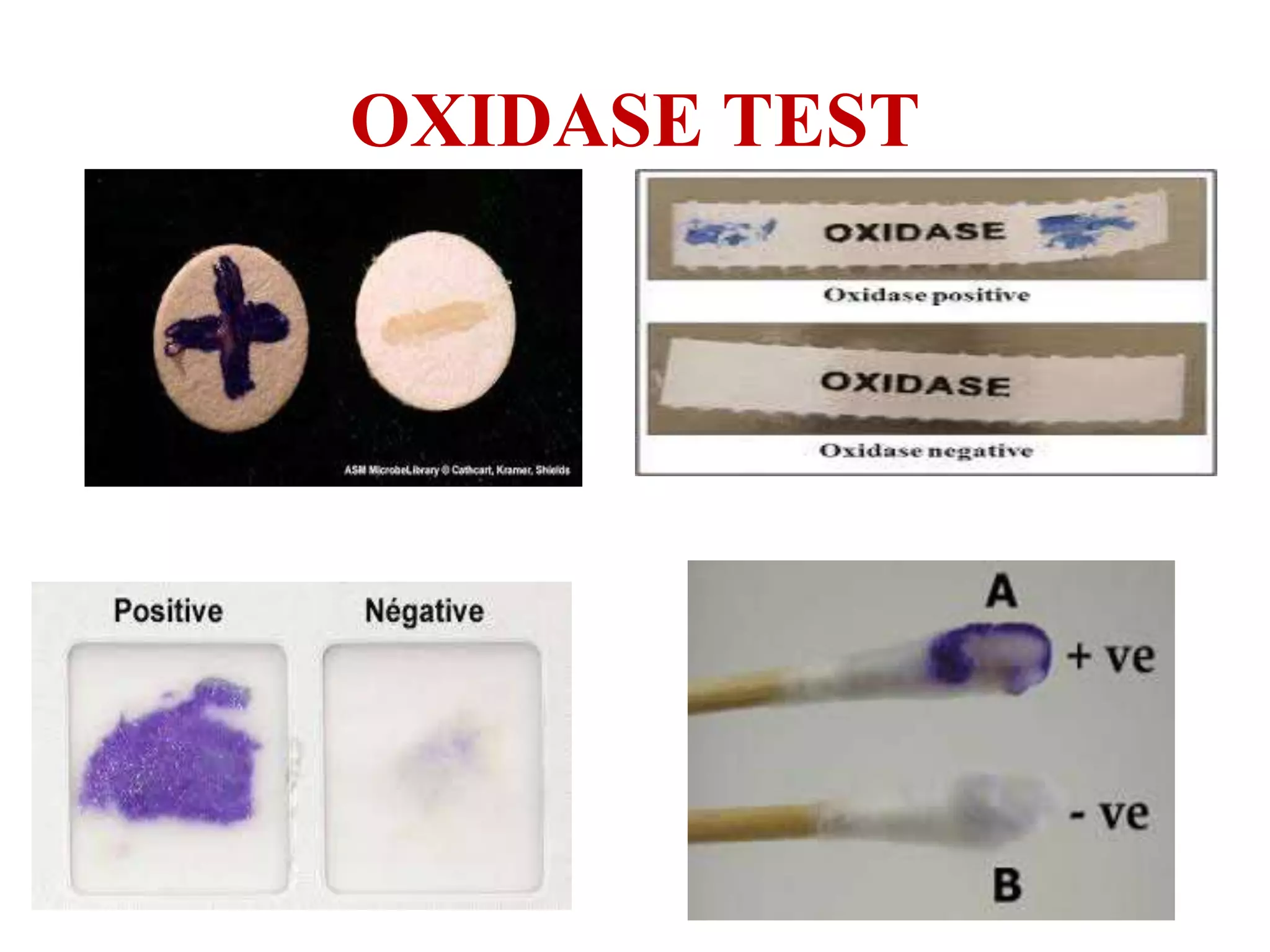
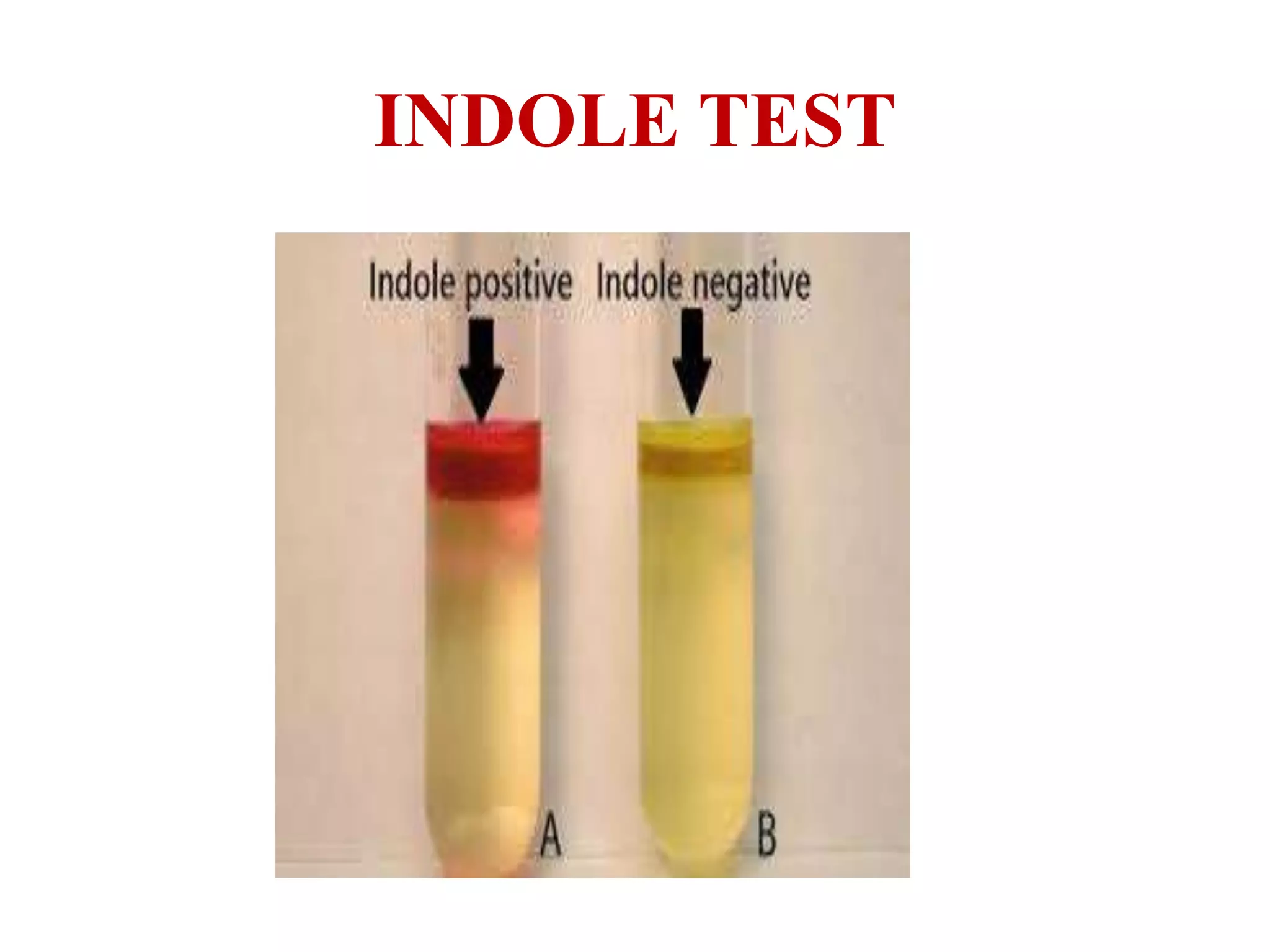
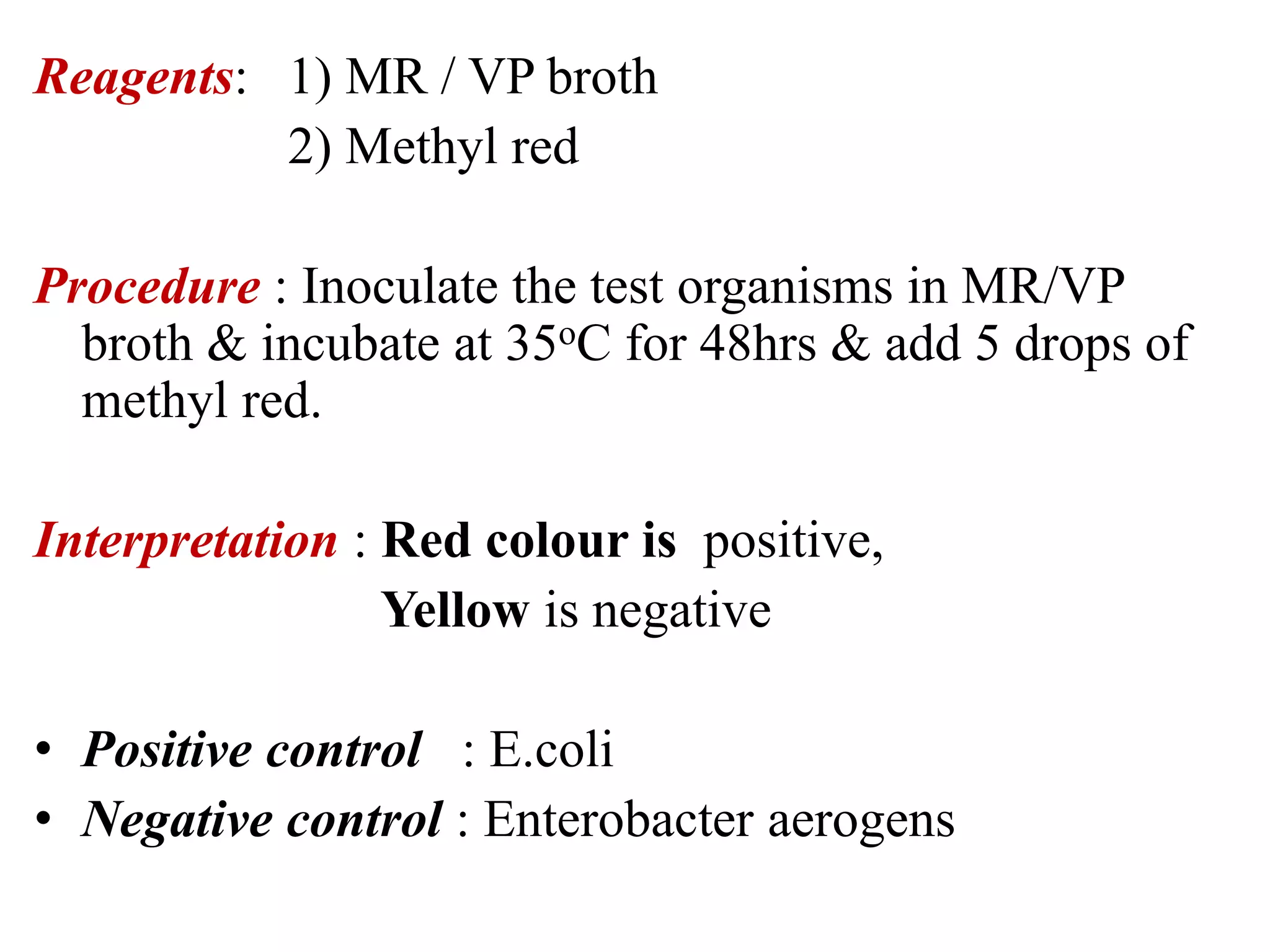
The document outlines various biochemical reaction tests used to identify bacterial species, including methods for catalase, oxidase, indole, methyl red, Voges-Proskauer, citrate utilization, urease, and triple sugar iron tests. Each test's principle, procedures, reagents, positive, and negative controls are detailed to guide laboratory identification. The document serves as a comprehensive reference for the biochemical testing of microorganisms.























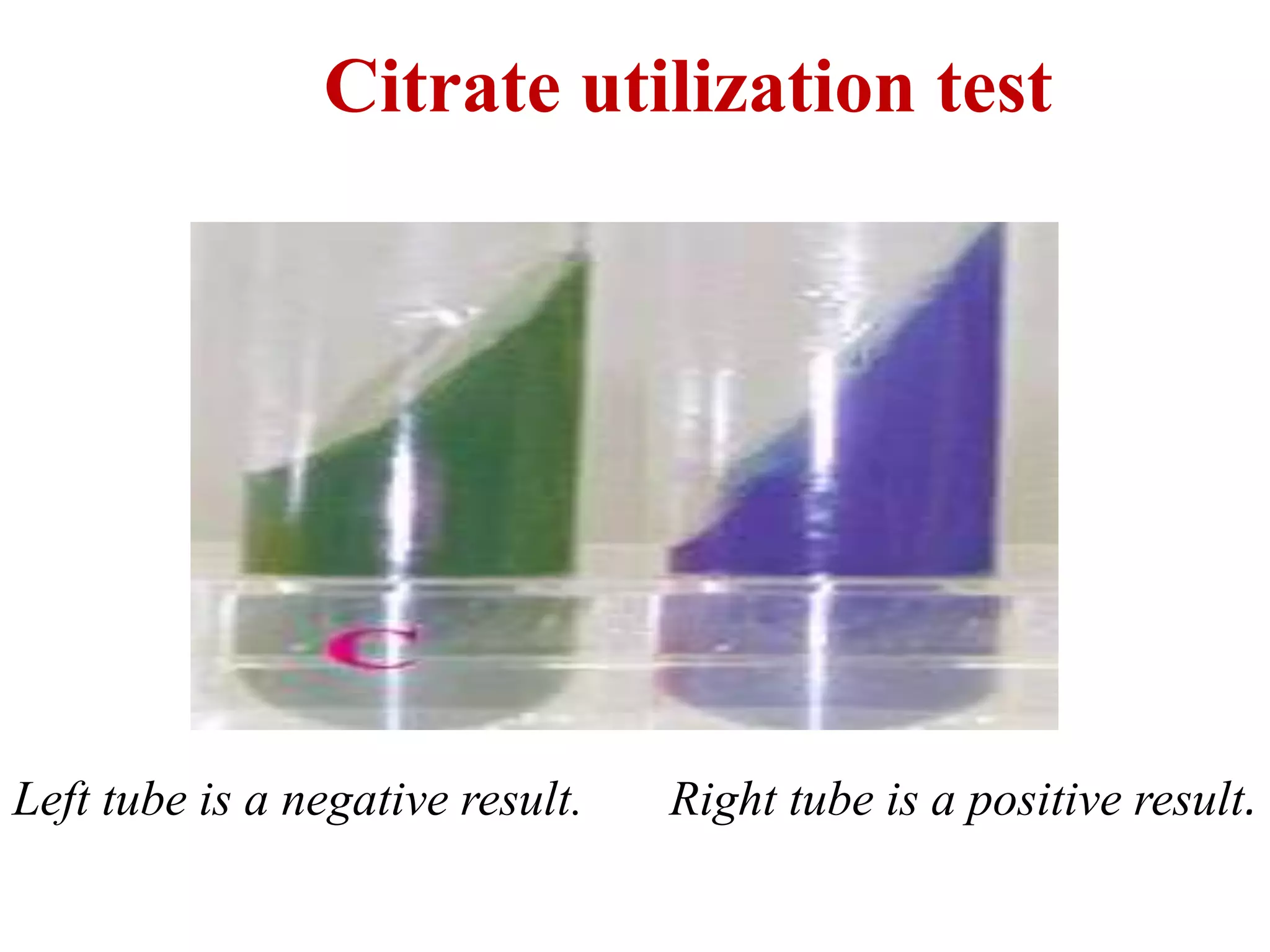
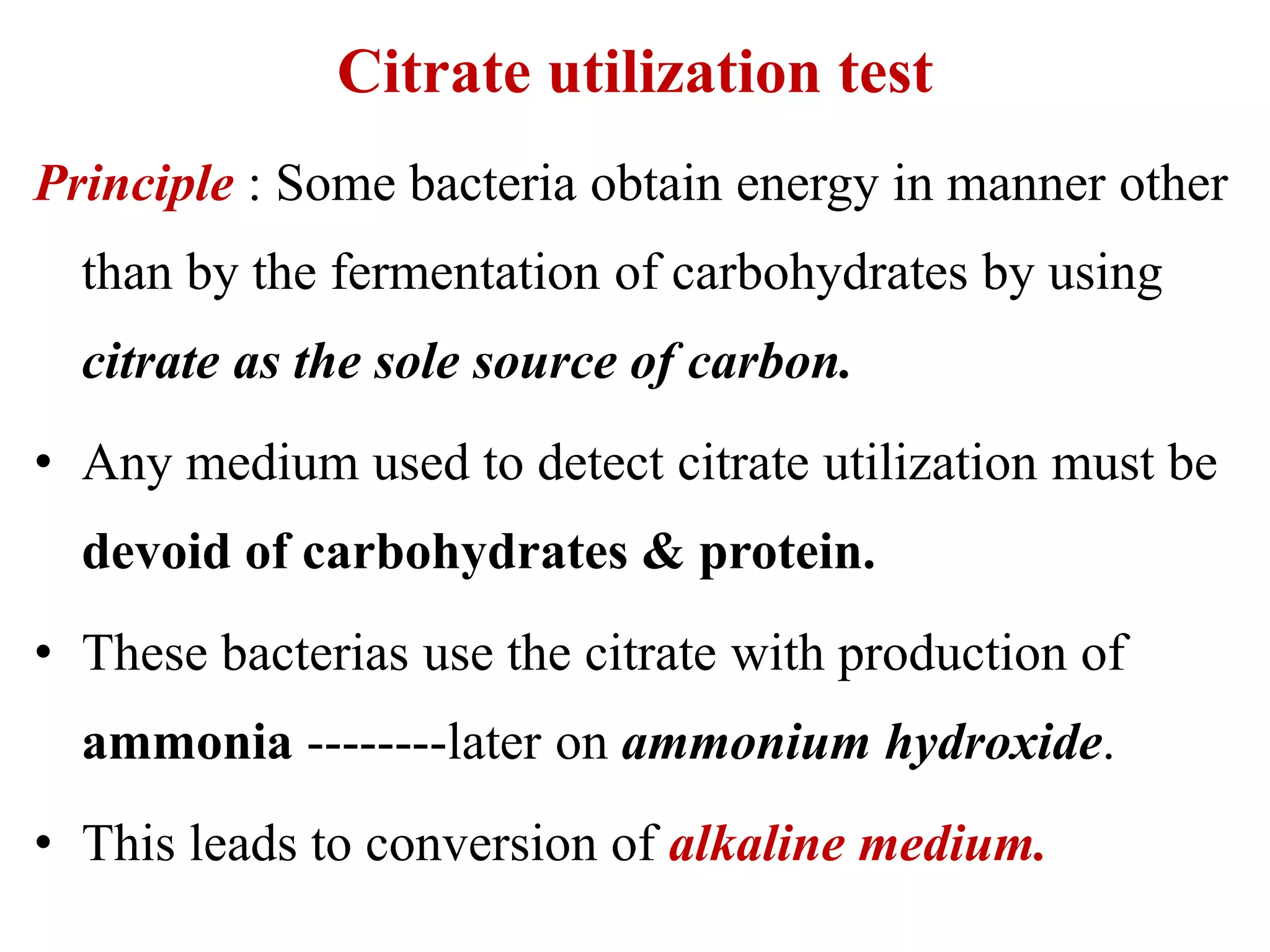
![Sodium citrate alkaline metabolic
products,increase PH
Bromothymol blue Bromothymol blue
[green-6.9PH] [Blue – PH: 7.6]
Reagents : 1. Simmon’s citrate media.
Procedure : Inoculate the citrate media with test organisms and
incubate at 35oC for 24-48hrs
Interpretation : Blue colour is positive, green is negative
• Positive control : Enterobacter aerogenes. Klebsiella spp.
• Negative control : E.coli](https://image.slidesharecdn.com/biochemicalreactionsnew-190412162327/75/Biochemical-reactions-new-30-2048.jpg)