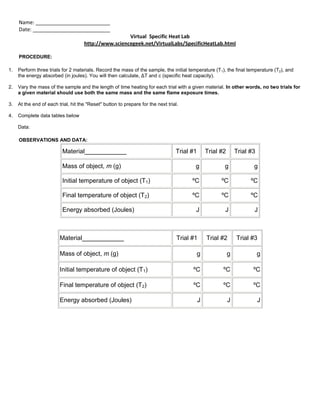
This virtual lab procedure involves performing three trials each for two different materials to determine their specific heat capacities. For each trial, the student will record the mass of the sample, its initial and final temperatures, and the energy absorbed. They will then calculate the temperature change and specific heat capacity, repeating the calculations for three trials of each material.