This document provides a summary of key concepts in physical chemistry in 3 sentences or less:
The document outlines fundamental concepts such as the laws of thermodynamics, kinetic molecular theory, ideal gases, real gases, phase diagrams, and transport properties. Key equations are presented for the ideal gas law, van der Waals equation of state, Maxwell-Boltzmann distribution, diffusion, heat transfer, and more. Definitions and examples are given for important terms like state functions, work, heat, entropy, heat capacity, compression factor, and diffusion coefficients.
![Dr. Lauth, University of Applied Sciences, Jülich Campus
Physical Chemistry
Formulary
The Laws of thermodynamics hold
William
Thomson
(Lord Kelvin;
1824 – 1907)
“when you can measure what you are speaking about,
and express it in numbers, you know something about it;
but when you cannot measure it,
when you cannot express it in numbers,
your knowledge is of a meagre and unsatisfactory kind”
E=E°+RT/F ln([Ox]/[Red])
k=Aexp(E/RT)
G=-RTln(K)
pV=nRT
r=k[A]a
[B]b
pi=xip*
dn/dt=-ADdc/dx
a=a[A]/([A]+K)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-1-320.jpg)

![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 2
Thermodynamic Process / Work and Heat
Process
A thermodynamic process is defined as a system
preceeding from an initial state to a final state.
Work and Heat are no state functions but
process quantities because they describe the
transition between states
Work W [J]
Convention: positive sign of W (W>0) indicates
that the system´s energy is increasing
The work accompanying a change in volume is
called „pV work“ = WVol
WVol = - p dVex
(pex[Pa]: external pressure)
Heat Q [J]
Convention:
positive sign of Q = endothermic process
negative sign of Q = exothermic process
Heat Capacity C [J/K]
Heat capacity is the heat required to raise the
temperature of a substance by 1 K
Index „p“: process occuring at constant pressure
Index „V“: process occuring at constant volume
Td
Q
=C;
Td
Q
=C V
Vp
p
Specific and Molar Heat Capacity c
[J/(kg K)] , Cm [J/(mol K)]
c =
C
m
c =
C
m
p
p
V
V
C =
C
n
C =
C
n
p,m
p
V,m
V
(m[kg]: mass; n[mol]: moles)
Examples (25°C / 1 bar):
Substance H2 N2 H2O C2H5OH Ag Fe Si SiO2 CDia
(g) (g) (l) (l) (s) (s) (s) (s) (s)
Cp,m [JK-1
mol-1
] 28,82 29,12 75,29 111,5 25,35 25,1 28,09 44,4 6,113
Standard State (index °) definitions:
- For a gas the standard state is a pressure of 1 bar (p°)
- For a substance present in a solution, the standard state is a concentration of 1 mol/L (c°)
- For a liquid or solid substance, the standard state is a molar fraction of 1 (pure substance)
nitial state Final state
Ti pi Vi Tf pf Vf
Work
Heat](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-3-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 3
Ideal Gases
Equation of State of an Ideal Gas
TRn=Vp
(p [Pa]: pressure; V[m³] volume; T [K] temperature; n[mol] moles; R = 8,314 J/(mol K) universal gas constant)
GAY-LUSSAC´s Law (CHARLES´s Law)
The volume of a given sample of gas at constant
pressure is directly proportional to the temperature
in Kelvins
T
K273,15
V
=V 0
BOYLE-MARIOTTE´s Law
The volume of a given sample of gas at constant
temperature varies inversely with the pressure
AVOGADRO´s Hypotheses
Equal volumes of gases at the same temperature
and pressure contain the same number of particles
Examples (273 K; 101325 Pa = 1 atm):
Gas (ideal) N2 H2 O2 He CO2 NH3 C2H6
(g) (g) (g) (g) (g) (g) (g)
Vm [10-3
m3
mol-1
] 22,414 22,40 22,43 22,39 22,43 22,26 22,40 22,17
DALTON´s Law of Partial Pressures
For a mixture of gases in a container, the total
pressure exerted is the sum of the pressures that
each gas would exert if it were alone
py=pp=p total
i
total iii
(y [mol/mol]: molar fraction of component „i)
Composition of dry air (mean molar mass M = 28,96 g/mol):
N2 O2 Ar CO2
Vol.-%
(Weight %)
78,09
(75,52)
20,95
(23,14)
0,93
(1,29)
0,03
(0,05)
const.=Vp
const.)=p(T,const.=V=
n
V
m](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-4-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 4
Kinetic-Molecular Theory I
Postulates of the kinetic molecular theory of ideal gases:
The volume of the individual particles can be assumed to be negligible
The particles are assumed to exert no forces on each other
Pressure p [Pa] and
Temperature T [K] of a Gas
The collisions of the particles with the walls of the
container are the cause for the pressure exerted by
the gas
p =
1
3
N
V
m v1 2
(N [] : number of molecules; m1
[kg] : mass of a molecule; v² [m²/s] : mean-square
velocity)
The average kinetic translational energy of a
collection of gas is assumed to be directly
proportional to the Kelvin temperature of the gas
trans
Atransm,B
N=UTk
2
3
=trans EE
(kB = 1,38110-23
J/K : BOLTZMANN constant; R = 8,314 J/(mol K): gas constant ; NA
= 6,022 1023
1/mol : Avogadro´s number; N []: number of particles; Utrans [J] internal
translational kinetic enrergy ; Um [J/mol] : molar translational kinetic energy)
MAXWELL-BOLTZMANN Distribution Law
d N
N d v
=
2 M
R T
v exp -
M v
2 R T
2
2
3
2 M
R
=
m
k
1
B
(N [] : number of particles; M [kg/mol]: molar mass; R = 8,314 J/(mol K): gas constant; v [m/s] : velocity)
average velocity
v =
8 R T
M
most probable velocity
v =
2 R T
M
h
square root of the mean square velocity
v =
3 R T
M
2
Examples:
Gas N2 He H2 Cl2 CO2 NH3 O2 Hg Ar
v [m s-1
] (298 K) 475 1256 1770 298 379 609 444 177 398](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-5-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 5
Kinetic-Molecular Theory II
Cross Section of Collision [m²]
= 4 r2
Mean Free Path ̅ [m]
The average distance a molecule in a given gas
sample travels between collisions with other
molecules
̅
√
(r [m] : (mean) radius of particle, C = N/V [1/m³]: concentration of particles; p[Pa] : pressure; T [K] : temperature; kB = 1,38110-23
J/K : BOLTZMANN constant)
Collision Frequency zt [1/s] of
Intermolecular Collisions
̅
̅
(v [m/s] mean velocity, ̅ [m] mean free path)
Collisions of Gas Particles with the
Container Wall zW [1/(m²s)]
z =
1
4
C vW
(v [m/s] mean velocity, C [1/m³] = N/V concentration of
particles )
Examples (293 K, 1 bar):
Gas N2 H2 CO2 O2 Ar
m [10-10
m] 600 1123 397 647 635
zt [109
s-1
] 5,07 10,0 6,1 4,4 4,0
BOLTZMANN Distribution and Barometric Distribution Law
The ratio of the populations in two energy states
E1 and E2 (distribution of thermal energy on
energy states)
N N
E
E E2 1
exp
k TB
Applied to gas molecules in earth atmosphere
p ph
0
exp
M g h
R T
(M [kg/mol]: (mean) molar mass; h [m]: height; g = 9,81 m/s² :
gravitational acceleration; R = 8,314 J/(mol K): gas constant)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-6-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 6
Real Gases I
Compression Factor Z []
Z =
p V
R T
=
p V
n R T
m
Virial Equation
p V = A + B p + C p + D p + ...m
2 3
2. Virial Coefficient B [m³/mol] and Boyle-
temperature TB [K]
B b
RT
a
a
T =
R b
B
Examples :
Gas N2 CO2 Ar H2 O2
B[10-6
m3
mol-1
](273K) -10,5 -149,7 -21,7 13,7 -22,0
B[10-6
m3
mol-1
](373K) 6,2 -72,2 -4,2 15,6 -3,7
VAN-DER-WAALS Equation of State
p +
V
V - b = R T
m
2 m
a
Covolume b [m³/mol]
b = 4 N
4
3
rA
3
Simplified VAN-DER-WAALS Equation
p V = RT + -
R T
pm b
a
(a/V²m [Pa]: internal pressure; Vm [m³/mol]: molar volume; b [m³/mol]: covolume; R = 8,314 J/(mol K): Gas constant) ,b [m³/mol]:
covolume; NA = 6,022 1023
1/mol : Avogadro´s number; r [m] radius of gas particle)
Examples:
Substance Ar N2 CO2 H2O CH4 C2H6
a [m6
Pa mol-2
] 0,1363 0,1408 0,364 0,5536 0,23 0,5562
b [10-5
m3
mol-1
] 3,219 3,913 4,267 3,049 42,9 6,38
repulsion
attraction
repulsion
attraction repulsion
attraction
repulsion
attraction](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-7-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 7
Real Gases II
Critical Constants pk, [Pa], Tk [K], Vm,k [m³/mol]
b3=V km,
2
27
=pk
b
a
R27
8
=Tk
b
a
)Z=(
8
3=
TR
V
k
k
km,kp
Examples:
Substance N2 H2 CO2 H2O CH4 C2H6 C5H12
Tk [K] 126,2 33,3 304,2 647,4 190,7 305,4 425,2
pk [M Pa] 3,394 1,297 7,387 22,119 4,63 4,884 3,8
Vm,k [10-6
m3
mol-1
] 90,1 65,0 94,0 56,0 98,7 148,0 255
JOULE-THOMSON Coefficient J-T [K/Pa] and Inversion Temperature Ti [K]
When a non-ideal gas is forced through a membrane or porous plug, its temperature changes
A gas cools on expansion at temperatures below Ti , above Ti a gas warms on expansion
=
2
R T
-
CJ-T
p,m
a
b
T =
2
Ri
a
b
Examples:
Gas N2 He CO2 N2 H2
J-T [K bar-1
] (300 K) 0,25 -0,059 1,1 0,25
Ti [K] 621 40 1500 621 202](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-8-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 8
Transport Properties I - Diffusion and Heat Transfer
Diffusion (FICK´s first and
second Law)
The rate of diffusion (the diffusive flux dn/dt) is
proportional to the concentration gradient dc/dx
The rate of accumulation (or depletion) of
concentration is proportional to the local
curvature of the concentration gradient d²c/dx².
dx²
d²c
D
dt
dc
xd
cd
-=
A
n
D
for a perfect gas (self-diffusion)
(A[m²]: area; D [m²/s]: diffusion coefficient)
Examples:
hydrogen ethanol Sugar chloride-ion sodium-ion silver
in (T) air(301K) H2O(298 K) H2O (298 K) H2O (298 K) H2O (298 K) copper(900 K)
D[m2
/s] 710-5
1,08.
10-9
5,21.
10-10
2,03.
10-9
1,33.
10-9
1,38.
10-15
Randow-walk Equation
(EINSTEIN-SMOLUCHOWSKI
Equation)
x² is the mean square distance traversed by a
molecule in time t
x² = 2 D t
Heat Transfer by Conduction
(FOURIER´s Law)
The rate of flow of heat dQ/dt is proportional to
the temperature gradient dT/dtx
xd
Td
-=
A
Q
W
cCv:gasdiatomicafor mV,mW
[A [m²]: area; w [W/(Km)] : thermal conductivity]
Examples (273 K):
Substance N2 H2 Xe H2O H2O Cu glas wood soil concrete
(g) (g) (g) (l) (s) (s) (s) (s) (s) (s)
w [WK-1
m-1
] 0,024 0,1682 0,0052 0,600 0,592 393 ~0,7 ~0,1 ~0,35 ~1,3](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-9-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 9
Transport Properties II - Viscosity
Viscosity
(NEWTON´s Law of Viscous Flow)
The frictional force F (resisting the relative
layers in the fluid) is proportional to the area A
and to the velocity gradient dvy/dx
(applies only to laminar flow)
xd
vd
-=
A
Y
V
F
Application: rotating-plate viscosimeter
v
2
1
=:gasperfectafor mV
(F [Pa]: frictional force; A [m²]: area; = F/A [Pa]: shear
stress; dvy/dx [1/s]: shear rate)
Examples:
Substance N2 (g) H2 (g) Ar(g) H2O (l) EtOH (l) Hg (l) H2SO4 (l)
298K 298K 273 K 293K 273K 298K 298K 293 K 298 K
V [mPas] 0,018 0,0087 0,021 0,0223 1,8 1,009 1,19 1,55 21,6
HAGEN-POISEUILLE Equation
The viscosity V can be calculated from
measurements of the rate of flow dV/dt in a tube
of known dimensions (l, r), if the pressure
difference p is known
d V
d t
=
r
l
p
4
V
8
Application: extrusion viscosimeter
STOKES´s Law
The frictional force Fd opposing the motion of a
large spherical particle of radius r moving at
speed v through a solvent of viscosity V is
given by STOKES´s law
F = 6 r vR V
Application: falling ball viscosimeter](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-10-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 10
The First Law of Thermodynamics
Internal Energy U [J], including
The kinetic energy of motion of the individual
molecules (rotation, vibration, translation)
The potential energy that arises from
interactions between molecules
The kinetic and potential energy of the electrons
within the individual molecules (chemical
bonds, etc.)
U = + E )kin pot
(E
First Law of Thermodynamics
The energy of the universe is constant
Applied to closed systems:
change of internal energy =
heat absorbed by the system +
work done on the system
Enthalpy H [J]
H = U + p V
Processes at Constant Volume
Increase of internal energy U of a system at
constant volume is equal to the heat QV that is
supplied to it
V
V
T
T
T
U
C
TdC=U=Q
2
1
21
T
VTV
Processes at Constant Pressure
Increase of enthalpy H of a system at constant
pressure is equal to the heat Qp that is supplied
to it
p
p
T
T
T
CH
H
TdC==Q
2
1
21
T
pTp
Phase Transitions
(at constant pressure)
The enthalpy change that occurs to melt a solid
at its melting point =
Molar enthalpy of fusion: fH = Qp,sl / n
Molar enthalpy of vaporization:vH = Qp,lg / n
Examples:
Substance Ar H2O C6H6 Ag
fH [kJ/mol] (at TE) 1,188 (83,8 K) 6,008 (273,1 K) 10,59 (278,6 K)) 11,3 (1234 K
vH [kJ/mol] (at TS) 6,51 (87,3 K) 40,656 (373,1 K) 30,8 (353,2 K) 250,6 (2436 K)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-11-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 11
Thermochemistry I - Basics
Stoichiometric Equation of a Chemical Reaction
A B C DA + B C + D
A, B: reactants C, D: products
Stoichiometric Coefficients i reactants < 0 products > 0 i i = 0
Extent of Reaction [mol] d = d ni / i ( = 0: reactants only; =1 products only)
Enthalpies of Formation H°f,i [J/mol]
Change in enthalpy („Heat“) that accompanies the formation of 1 mole of a compound from its elements
(with all substances in their standard state)
Examples (298 K, 1 bar):
compound N2 H2O H2O CO2 CO NH3 NO C2H4 C6H6
(g) (l) (g) (g) (g) (g) (g) (g) (l)
H0
B [kJ mol-1
] 0 - 285,84 -241,83 -393,51 -110,52 -46,19 90,37 52,3 49,03
more examples see appendix
Enthalpy of Reaction RH° [J/mol]
Heat associated with a chemical reaction at
constant pressure rH can be calculated from
the enthalpies of formation of the reactants and
products
Enthalpy and energy changes are considered
with reference to the extent of reaction;
stoichiometric equation must be specified
i
if,
o
i
o
H=H r
0
reactantsf,
0
productsf, H-H=
Energy of Reaction RU [J/mol]
Heat associated with a chemical reaction at constant volume; rU can be calculated from rH
r r r r r rH = U + (p V) H = U + R T ngaseous
(R = 8,314 J/(mol K) : gas constant Rngaseous: change in moles of gaseous products and reactants)
HESS´s Law
The enthalpy of reaction is not
dependent on the reaction pathway
RH = 0
d H = 0
](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-12-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 12
Thermochemistry II - Calorimetry
Enthalpy of Combustion cH [J/mol]
Amount of heat produced for the complete combustion of a substance (at constant pressure)
Examples :
compound H2 CH4 C2H4 C4H10 CH3OH C8H18 C12H22O11
(g) (g) (g) (g) methanol(l) i-octan(l) saccharose(s)
cH [kJ mol-1
] - 286 -890 -1411 - 2877 -726 -5461 -5645
Measurement of Enthalpy Changes (Calorimetry)
Direct measurement of rH or indirect calorimetry (application of Heß´s law)
Indirect calorimetry: determination of enthalpies of formation HB,i from enthalpies of combustion cH
icproductscombustionf,, H-H=ifH
KIRCHHOFF´s Law
Temperature dependence of enthalpies of
reaction can be calculated from the heat
capacities of the reactants and products
H
T
= C
H = H + C dT
i p,i
i
T T
T
T
i p,i
i
2 1
1
2
R
R R
Enthalpy of Atomization - Hf,at [J/mol]
Heat to be supplied at constant pressure in order
do dissociate all the molecules into gaseous
atoms
atomsfgifiatf HHH ,)(,,,
Bond Enthalpy Hbond
The bond enthalpie or bond strenth is an average quantity
bondiatf HH ,,
Examples:
Bond O-H C-H C-C C=C CC C-O C-N C=O N-H
(ketone)
Hbond [kJ mol-1
] -463 -416 -340 -615 -815 -340 -296 -750 -390](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-13-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 13
Second Law of Thermodynamics - Entropy
Second Law of Thermodynamics
The entropy of the universe tends towards a
maximum (while the energy of the universe is a
constsant)
Suniverse 0
In any spontanous irreversible process (occuring
without outside intervention) there is always an
increase in the entropy of the universe
Suniv., irrev. > 0
In reversible processes the entropy of the
universe remains constant
Suniv., rev. = 0
Entropy S [J/K]
measure of randomness or disorder; increase in entropy means an increase in disorder
Microscopic Definition of Entropy (BOLTZMANN´s Equation)
entropy is related to thermodynamic probabiltiy W, the number of microstates corresponding to a given state
(including both position and energy)
S = k ln(W)B
(kB = 1,38110-23
J/K: BOLTZMANN constant; W []: thermodynamic probability)
Macroscopic Definition of Entropy
Change S (CLAUSIUS)
S =
Q
T
d S =
Q
T
rev rev
(Qrev [J] heat absorbed or released during a reversible process; T [K] temperture)
Third Law of Thermodynamics
(NERNST´s Heat Theorem)
The entropies of a perfect crystal at 0 K is zero
S = 0crystalline, pure, 0 K
Absolute Entropies S°i [J/(mol K)]
The more complex the molecule, the higher the
standard entropy value
Examples: (1 bar, 298 K)
Substance Si N2 N2O4 H2O H2O SiCl4 SiF4 CO2 C6H6
(s) (g) (g) (l) (g) (l) (g) (g) (l)
S0
i [J K-1
mol-1
] 18,82 191,5 304,3 69,94 188,72 239,32 282,14 213,64 172,8
more examples see appendix](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-14-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 14
Entropy and Energy Conversion
Temperature Dependence of Entropy
S =
C
T
d TT T
p
T
T
1 2
1
2
(Cp [J/(mol K)] : molar heat capacity)
Entropy Changes Associated with
Changes of Aggregation State
entropy of fusion:
f
H
S =
T
f
.E
;
entropy of vaporization:
v
v
S
S =
T
H
Examples:
Substance Ar N2 O2 Cl2 H2O CH3OH C6H6 CH3COOH
fS [J/(K mol)] (at TE) 14,17 11,39 8,17 37,22 22,00 18,03 38,00 40,4
vS [J/(K mol)] (at TS) 74,53 75,22 75,63 85,38 109,0 104,6 87,19 61,9
Concentration Dependence of Entropy (Expansion; Dillution)
S = n R ln
V
V
V V
2
1
1 2
(n [mol] :moles; R = 8,314 J/(K mol): gas constant)
Entropy Changes in Chemical Reactions RS° [J/(K mol)]
rS can be calculated from the absolute entropies of the reactants and products
Stoichiometric equation must be specified
RS = S = S -0
i i
0
i
product
0
reactant
0
CARNOT Cycle
The efficiency C of the reversible CARNOT
engine can be defined as the work done by the
system during the cycle Wrev, divided by the
heat absorbed at the higher temperature Q1
Q
W
=
1
rev
C
T
T-T
=
Q
W
1
12
1
rev
0WQQ rev21 ](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-15-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 15
Free Enthalpy and Equilibrium
Free Enthalpy (GIBBS Energy) G [J]
At constant temperature and pressure, systems
tend to move toward a state of minimum
GIBBS energy G H - TS (= second law of
thermodynamics for constant T and p)
rG = G/ is a measure for the tendency of
a reaction to occur; the more negative the
value of rG, the further a reaction will go to the
right to reach equilibrium
rG can be calculated from standard reaction free enthalpy rG° and reaction ratio Qr
R R RQG = G + RT ln0
i
iR a=
][Reactants
[Products]
=Q i
Reactants
Products
The standard reaction free enthalpy rG° can be calculated from standard reaction enthalpy rH° and
standard reaction entropy rS° (sometimes called GIBBS-HELMHOLTZ equation)
R R RG = H - T S
Equilibrium of a reaction is established when
rG = 0 (minimum GIBBS energy); the reaction
ratio has a particular value - the thermodynamic
equilibrium constant KGG
i
i
GG
GG
a=
][Reactants
[Products]
=K i
Reactants
Products
GG
The equilibrium constant K can be calculated from the standard reaction free enthalpy rG°
R
G = - R T ln Ko
K = exp -
G
R T
o
R
Examples: (298 K)
reaction N2 + 3 H2 2 NH3 H2O H+
+ OH -
K 6,8105
bar-2
1,010-14
mol²/l²
reaction CH3COOH CH3COO-
+ H+
AgCl Ag+
+ Cl -
K 1,810-5
mol²/l² 1.610-10
mol²/l²](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-16-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 16
Shifts of Equilibrium
Exergonic Reations Endergonic Reactions
RG° < 0 RG° > 0
KGG > 1 KGG < 1
Temperature Dependence of Equilibrium Constants
ULICH´s approximation : rG° for temperatures other than 298 K can be calculated from rH°- and rS°-
values for 298 K
R R R
G (T) = H (298 K) - T S K)o o o
(298
Possible Combinations of H° and S° for a Process
RH° < 0 RS° > 0 exergonic (spontaneous) at all temperatures
RH° < 0 RS° < 0 exergonic at T < RH°/RS°
RH° > 0 RS° > 0 exergonic at T > RH°/RS°
RH° > 0 RS° < 0 not exergonic at any temperature
VAN´T HOFF´s Equation
A plot of ln{K} against 1/T will have a slope
equal to H°/R
ln K = -
H
R
1
T
+
S
R
o o
R R
(T [K]: temperature; R = 8,314 J/(K mol) : gas constant)
Examples:
Reaction N2 + 3 H2 2 NH3 N2 + O2 2 NO
T 298 K 400 K 500 K 1000 K 3000 K
K 6,8105
bar-2
41 bar-2
3,510-2
bar-2
6,810-9
0,017
LE CHATELIER/BRAUN Principle
If a change in conditions (a „stress“) is imposed
on a system at equilibrium, the equilibrium
position will shift in a direction that tends to
reduce that change in conditions
process „stress“ shift
RH > 0 increase in temperature product yield higher
RH < 0 increase in temperature product yield lower
RV < 0 increase in pressure product yield higher
RV > 0 increase in pressure product yield lower](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-17-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 17
Phase Equilibria of One-Component Systems
Vapor Pressure [Pa]
Vapor pressure = the pressure of the vapor over
a liquid at equilibrium
At equilibrium, the rates of condensation and
evaporation are equal (dynamic equilibrium)
Examples:
Substance H2O(s) (s/l) H2O(l) (vH = 41 kJ/mol ) | Hg(l) (vH = 59 kJ/mol)
T [°C] -10 0,0 10 25 100 150 200 | -10 25 100
p [mbar] 2,5 6,0 12,3 31,7 1 013 4 760 15 551 | 8,810-5
110-3
0,35
ANTOINE´s Equation
Empirical equation for vapor pressure change
with temperature
(p [bar] : vapor pressure in bar; 1 bar = 100 000 Pa; T [°C]
temperature in °C ; A, B, C ANTOINE constants)
examples:
Substance benzene ethanol toluol water
A 4,2144 3,9251 4,0899 4,9513
B 1314,90 912,01 1348,21 1575,61
C 232,40 154,35 219,55 218,62
CLAUSIUS-CLAPEYRON
Equation
The CLAUSIUS-CLAPEYRON equation represents
the vapor pressure change with temperature
Enthalpy (“heat”) of vaporization vH is
assumed to be independent of temperature
A “CLAUSIUS-CLAPEYRON-plot” (ln(p) versus
1/T) results in a straight line with a (-vH /R) -
slope
[ ( )]
(T1 / T2 [K] temperature corresopoding to vapor pressure p1 / p2; vH
[J/mol]: enthalpy of vaporization; R = 8,314 J/(mol K): gas constant)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-18-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 18
Surface Tension
Definition of Surface Tension
[J/m² = N/m]
Surface Tension : the work dW necessary to
increase the surface area by dA
Ad
Wd
=
L
F
=
Examples (20 °C)
Substance H2O (l) Ethanol (l) glycerol (l) Hg (l)
[mN m-1
] 72,8 22,3 63,0 480
YOUNG Equation and Meniscus Angle
LG
SL-
=)(cos
SG
([°]: meniscus angle ; SG [N/m] surface tension between solid phase and gas)
Examples (20 °C)
(l) C6H6 H2O H2O Hg Hg
(s) glas glas wax steel glas
6° 0° 105° 154° 140°
LAPLACE Equation
The pressure on the inside of a bubble (radius r)
must be higher than on the outside
r
2=p
convexconcave p-p=p
( [N/m] surface tension; p [Pa] Pressure difference; r [m]
radius of the surface curvature)
Capillarity
Spontaneous rising of a wetting liquid in a
narrow tube to the height h
rg
)(cos2
=h
(: meniscus angle; [kg/m³] density; g = 9,81 m/s² :
gravitattional acceleration; r [m]: radius of the capillary)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-19-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 19
Two-Component Systems - Ideal Solutions
Ideal Solutions
Mixture of component (A) = solvent and
component (B) = solute
Ideal solution: Inter molecular attractions of
(A) for (A) and of (B) for (B) are the same as
the attraction of (A) for (B) { interaction (A
B) interactions (AA / BB) }
RAOULT´s Law
Vapor pressure of solvent p is equal to its mole
fraction in the solution x multiplied by the vapor
pressure of the pure solvent p° (ideal solutions).
(p
A
[Pa] : vapor pressure of component A in sulution; p
A
*
[Pa] : vapor pressure of
pure component A; x
1
[]: mole fraction of component 1)
HENRY´s Law
The molar fraction of a gas dissolved by a given
volume of solvent at constant temperature, is
propotional to the pressure of the gas in
equilibrium with the solution.
(pB [Pa]: vapor pressure of component B (gas) in solution; kH
[Pa] : Henry´s constant; x2 []: molare fraction of component 2)
Examples (25 °C)
Component „2“ (gas) N2 O2 CO2 H2 CH4 N2 CO2 H2 CH4
Solvent „1“ H2O H2O H2O H2O H2O C6H6 C6H6 C6H6 C6H6
kH [GPa] 8,68 4,4 0,167 7,12 0,0419 0,239 0,0114 0,367 0,0569
Vapor Pressure and Boiling Point of two Liquids obeying Raoult´s Law
The total vapor pressure in a binary solution will be
intermediate between the vapor pressures of the
two pure components (no maximum or minimum in
p-x-diagram)
The boiling points of all the mixtures are inter-
mediate between the boiling points of the pure
components](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-20-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 20
Colligative Properties
A colligative propery is a property of dilute solutions that depends on only the number of solute molecules and
not on the type of species present
Boiling Point Elevation
Boiling point elevation is a colligative propery
The molality b is the number of moles of solute
per kilogram of solvent in a solution
(bB [mol/kg] : molality ; i[]: VAN´T HOFF factor; Keb [K kg/mol]:
ebullioscopic constant)
Freezing Point Depression
Freezing point depression is a colligative
property (RAOULT´s second law)
(bB [mol/kg] = nB/mA: molality ; i []: VAN´T HOFF factor; kkr [K
kg/mol]: cryoscopic constant)
Examples :
Solvent H2O C6H6 cyclohexane camphen acetic acid CS2 CCl4
keb [K kg/mol] 0,514 2,64 2,75 6,09 3,07 2,37 4,95
Kkr [K kg/mol] 1,86 5,07 20,2 40,0 3,90 3,8 30
Osmotic Pressure [Pa] (VAN´T HOFF Equation)
Osmosis: the flow of solvent into a solution
through a semipermeable membrane (permeable
for solvent molecules only)
Osmotic pressure : the pressure that must be
applied to stop osmosis
Molarity c: moles of solute / volume of solution
(i[] : van´t Hoff factor; cB [mol/m³]: molarity; R = 8,314 J/(mol
K) : gas constant)
Examples:
Solution 0,4 mol sugar in 1 kg water/ 20° human blood at 37 °C
[bar] 10,3 bar 7,7 bar
Dissociation (VAN´T HOFF Factor i)
van´t Hoff Factor i: the number of particles in
solution formed from one molecule
( )
(+,- []: number of cations/anions formed from one molecule; z+,-
[]: charge of cation / anion; []: degree of dissociation.)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-21-320.jpg)
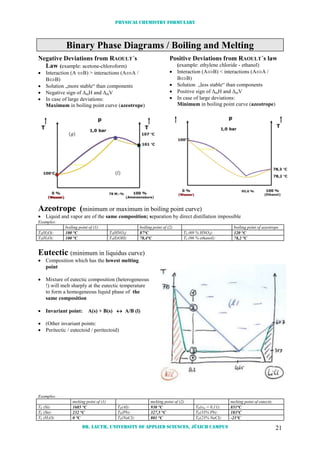

![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 23
Adsorption
Adsorption: the collection of one substance on the surface of another
Surface Concentration a [mol/g] and
Fraction of Surface covered [ ]
adsorbent
speciesadsorbed
m
n
=a
layermomoa
a
LANGMUIR Adsorption Isotherm
Formation of a unimolecular layer at saturation
[ ]
[ ]
(K [Pa or M]: Langmuir constant; [A] [Pa] Pressure or [M] :
molarity)
Surface areas of the sorbent can be calculated
from a (spec. area = a
.
NA
.
Aadsorbed molecule)
In order to test the Langmuir isotherm it is best
to use a reciprocal plot (1/a versus 1/c or 1/p)
BET Adsorption Isotherm
Extension of the Langmuir treatment (by
BRUNAUER, EMMETT AND TELLER) to allow for
the physisorption of additional layers of
adsorbed molecules
monad,
*
monad,ad
*
*
VK
p/p1)-(K
+
VK
1
=
V)p/p-1(
p/p
(K []: BET-constante; p* :saturation vapor pressure; Vmon:
volumen that can be adsorbed as a monolayer)
FREUNDLICH Adsorption Isotherm
[ ]
In order to test the Freundlich isotherm it is
best to use a logarithmic plot (ln(a) versus
ln(c))
)ln()ln(ln(a) c ](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-24-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 24
Chemical Kinetics
Rate of Consumption and Formation ri [mol/(s m³)]
The change in concentration of a reactant or
product per unit time
[ ]
Rate of Reaction r [mol/(s m³)]
Rate Law, Rate Constant and Half Life
Rate law: an expression, which shows how the
rate depends on the concentrations of reactants
r = k f(c ,c ,c ...)A B C
often
( ) [ ] [ ] [ ]
The proportionality constant k is called the rate
constant; the exponents a,b,c.. are called the
order of the reactant
The form of the rate law (the values for k, a, b, c
...) must be determined by experiment
The integrated rate law relates concentration
to reaction time:
cA = f(t)
The time required for a reactant to reach half of
its original concentration is called the half life of
a reaction and is designated by the symbol t½
0st
order reaction](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-25-320.jpg)


![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 27
Influence of Temperature and Catalyst on Reaction Rates
ARRHENIUS Equation
The rate constant k shows an exponential
increase with temperature
k = A exp
E
R T
A
(A: frequency factor; EA [J/mol] activation energy; R = 8,314 J/(mol K) gas constant)
A plot of ln(k) against (1/T) gives a straight
line („ARRHENIUS plot“); the value of the activation
energy can be obtained from the slope of the line, which
equals -EA/R; the intercept can be used to determine A.
ln( )k = ln(A) -
E
R
1
T
A
Activation energy EA : for a reaction , mole-
cules must come together with at least EA in order
to surmount the energy barrier (threshold energy)
Frequency factor A (preexponential factor):
rate constant at very high temperture
Examples:
Reaction 2 N2O5 2NO2 + O2 cyclopropane propene sucrose fructose + glucose
1. order, gas phase 1. order, gas phase 2. order; aqueous solution
EA 88 kJ/mol 272 kJ/mol 107,9 kJ/mol
A 6,311014
1/s 1,581015
1/s 1,5 1015
dm³/(mol s)
Collision Theory and
Activated Complex
Molecules must collide to react
(reaction rate = number of successfull collisons)
The relative orientation of the reactants
must allow formation of any new bonds
necessary to produce products (steric factor =
fraction of collisions with effective orientation)
The collision must involve enogh energy
to form the activated complex (transition state)
Catalysis
A catalyst is a substance that increases the rate
of reaction without being consumed itself
A catalyst does not modify the overall
thermodynamics (H, G, S) of the reaction
A catalyst provides a new pathway with lower
activation energy for the reaction
Reaction 2 HI H2 + I2
catalyst (none) Au(s) Pt(s)
EA [kJ/mol] 184 105 59](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-28-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 28
Electrochemistry - Solutions of Electrolytes
Dissociation of Electrolytes
Strong electrolytes occur almost entirely as
ions in solution; degree of dissociation 1
Weak electrolytes are present only partially as
ions; << 1
K A K + A+ -
+ -
+
z
-
z
n = z = - ze + + - -
( [] dissociation number of cation / anion; z [] : charge number of
cation / anion; ne [] : elektrochemical number)
Conductivity E [S/m]
Product from conductance (G [S] = 1/R) and
cell constant (C = d/A)
A
d
R
1
=E
E
i=1=
E
( [m] : specific resistance; E [V/m]: electric field ; R [] resistance,
d[m] distance between electrodes; A[m²] area of electrode)
Molar Conductivity m and Equi-
valent Conductivity [Sm²/mol]
m
=
c
E
=
c n
E
e
(c [mol/L]: molarity; ne []: electrochemical number)
Examples (25 °C, unless otherwise stated):
Substance Cu Fe NaCl (l) KCl 1M KCl 0,1 M ZrO2/Y(s) H2O
850 °C 750 °C
E [S/cm] 580000 96100 3,66 0,098 0,00112 0,01 4,41.
10-8
KOHLRAUSCH´s Law of Independent Migration of Ions -
Ion Conductivities [Sm²/mol]
Each ion is assumed to make its own
contribution to the molar conductivity,
irrespecitve of the nature of the other ion with
which it is associated
Examples (infinite dilution):
Ion H+
Na+
K+
Ca2+
OH-
Cl-
NO3
-
SO4
2-
CH3COO-
[cm²/( mol)] 349,7 50,1 73,5 59,5 197,0 76,4 71,5 80,0 40,9](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-29-320.jpg)

![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 30
Electrochemistry - Conductivity
Ionic Velocity v [m/s] and Ionic Mobility u [m²/V s]
The mobility u of an ion is defined as the speed with which the ion moves under a unit potential gradient.
The mobility can be calculated from ion conductivities
v = u E v = u E+ + - -
= F u = F u+ -
(E [V/m]: electric field)
Examples (infinite dilution):
Ion H+
Na+
K+
Ca2+
OH-
Cl-
NO3
-
SO4
2-
CH3COO-
u [10-4
cm²/(Vs) 36,23 5,19 7,62 6,17 20,64 7,91 7,4 8,29 4,34
Variation of Equivalent Conductivity with Concentration
a) Strong Electrolytes
(KOHLRAUSCH´s Law)
Electrophoretic effect: the ion and its
ionic atmosphere move in different directions
Asymmetry effect: the ionic atmosphere
around a moving ion is not symmetrical
= - const. c
b) Weak Electrolyte (OSTWALD´s Dilution Law)
conductivity is proportional to the degree of
dissociation
= K = c
2
( )1
1
c
1
=
1
2
K
( []: degree of diss.; [Sm²/mol] : equivalent conductivity at molarity c
[M]; [Sm²/mol] : equivalent conductivity at infinite dilution)](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-31-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 31
Electrochemistry - Electrodes
Electron Transfer Reaction
Half-reactions occuring at the interface between an
electrode and the solution:
Oxidation (transfer of electrons to the metal) occurs
at the anode [Ox]anode + e e-
[Red] anode
Reduction (transfer of electrons to the electrolyte)
occurs at the cathode [Ox]cathode + e e-
[Red] cath
NERNST Equation - Electrode Potential at Zero Current
The potential of a single electrode Eredox is
dependent on concentration
red
ox
red
ox
e
o
redox
a
a
ln
F
TR
+E=
redoxE
(R=8,314 J/(mol K) : gas constant; e []: number of electrons in electron
transfer reaction; F= 96 485 C/mol : Faraday constant)
Standard Electrode Potentials E°
React. Cl2+2e-
2Cl-
Ag+
+e-
Ag Fe3+
+e-
Fe2+
Cu2+
+2e-
Cu Zn2+
+2e-
Zn
E°redox 1,36 V 0,80 V 0,77 V 0,34 V - 0,76 V
Combinations of Electrodes - Cell Potential E [V] („EMF“)
The cell potential at zero current (electromotive
force „emf“ E) is the potential difference
beween the cathode and the anode
The galvanic cell runs spontaneously in the
direction that give a positive value for E
The process of electrolysis involves forcing a
current throgh a cell to produce chemical
change for which the cell potential E is negative
E = E - Eredox,cathode redox, anode
S, H and G values can be calculated, if emf
measurements are made over a range of
temperature
R
G = - F Ee
R
S = F
E
T
e
R
H = - F E - T
E
T
e
(RH [J/mol] : enthalpy of reaction; RS [J/(K mol)] : entropy of reaction; RG [J/mol]
: free enthalpy of reaction; E [V]: electromotive force emf; e []: number of electrons
transferred for every unit of reaction; F= 96 485 C/mol : Faraday constant)
Reference Electrodes and Potentials:
electrode Diagram potential
standard hydrogen electrode (SHE); Pt/H2(1 bar), H+
(a= 1mol/l) E = 0,00 V
calomel electrode, saturated; Hg/Hg2Cl2, KCl (sat.) E = 0,241 V
silver chloride electrode, saturated; Ag/AgCl, KCl (sat.) E = 0,197 V
Galvanic Cell](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-32-320.jpg)
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 32
Electrochemistry - Electrolysis
FARADAY´s Law of Electrolysis
The mass of an element produced at an electrode is
proportional to the quantitiy of electricity passed
through the liquid and proportional to the
equivalent weight of the element
m =
I t M
F e
(m [kg]: mass; I [A] : current; t [s] : time; M [kg/mol] : molar mass;
F = 96 495 C/mol: FARADAY constant; e []: number of electrons in
electron transfer reaction)
Liquid Junction (Diffusion) Potential and Membrane Potential
At the liquid junction of two different electrolytes the ions have different mobilities t+ and t-. The movement of
the ions across this boundary generates a current flow and hence a potential (junction or diffusion potential
diff; membrane potential mem, if the junction is permeable only to one kind of ion)
2
1
+
c
c
ln)(t
Fz
TR
-= tdiff
2
1
c
c
ln
Fz
TR
-=mem](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-33-320.jpg)

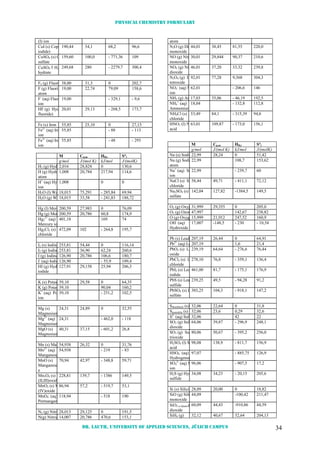
![Physical chemistry formulary
Dr. Lauth, University of Applied Sciences, Jülich Campus 35
Monosilane
SiF4 (g) Si-
tetrafluoride
104,08 73,6 -1614,9 282,14
SiCl4 (l) Si-
tetrachloride (
169,90 145,2 -640,15 239,32
SiCl4 (g) Si-
tetrachloride
169,90 -657,31 330,83
Snweiß (s) Tin118,69 26,99 0 51,5
Sngrau (s) Tin118,69 - 2,19 44,1
Sn2+
(aq) Tin ion118,69 - 10 - 20,5
Zn (s) Zink 65,37 25,40 0 41,59
Zn2+
(aq) Zink ion65,37 46 - 152,4 - 106,5
AVOGADRO number NA = 6,0221023
1/mol
Universal gas constant R = 8,3145 J/(mol K)
BOLTZMANN constant kB = 1,38110-23
J/K
Charge of a proton e = 1,6021810-19
C
FARADAY constant F = 96485,3 C/mol
Permittivity of vacuum 0 = 8,85410-12
C2
/(Jm)
Speed of light c = 2, 9979 108
m/s
PLANCK constant h = 6,6261 10-34
Js
Mass of an electron me = 9,10938 10-31
kg
Mass of a proton mp = 1,672610-27
kg
Acceleration of gravity g = 9,81 m/s2
Electrochemical Reduction Potentials
(p = p0
= 1 bar; c = c0
= 1 mol/L ; T = 298 K; aqueous solution)
[Ox] + e e-
[Red] E0
redox [V]
F2 (g) + 2 e-
2 F-
+ 2,85
S2O8
2-
+ 2 e-
2 SO4
2-
+ 2,0
PbO2(s) + SO4
2-
+ 4 H+
+ 2 e-
PbSO4 (s) +
2 H2O
+ 1,685
MnO4
-
+ 8 H+
+ 5 e-
Mn2+
+ 4
H2O
+ 1,491
PbO2 (s) + 4 H+
+ 2 e-
Pb2+
+ 2 H2O + 1,455
Ce4+
+ e-
Ce3+
+ 1,443
Au3+
+ 3 e-
Au (s) + 1,42
Cl2(g) + 2 e-
2 Cl-
+ 1,358
O2 (g) + 4 H+
+ 4 e-
2 H2O + 1,229
HCrO4
-
+ 7 H+
+ 3 e-
Cr3+
+ 4 H2O + 1,195
Br2 (l) + 2 e-
2 Br-
+ 1,065
AuCl4
-
+ 3 e-
Au (s) + 4
Cl-
+ 0,994
NO3
-
+ 4 H+
+ 3 e-
NO (g) + 2
H2O
+ 0,96
Ag+
+ e-
Ag (s) + 0,7996
Hg2
2+
+ 2 e-
2 Hg (l) + 0,798
Fe3+
+ e-
Fe2+
+ 0,770
C6H4O2 + 2 H+
+ 2 e- C6H4(OH)2 + 0,6995
I2 (s) + 2 e-
2 I-
+ 0,535
Cu+
+ e-
Cu (s) + 0,522
O2 (g) + 2 H2O + 4 e-
4 OH-
+ 0,401
Cu2+
+ 2 e-
Cu (s) + 0,340
AgCl (s) + e-
Ag (s) + Cl-
+ 0,2223
[Ox] + e e-
[Red] E0
redox [V]
SO4
2-
+ 4 H+
+ 2 e-
H2SO3 (aq) +
H2O
+ 0,20
Sn4+
+ 2 e-
Sn2+
+ 0,15
S(s) + 2 H+
+ 2 e-
H2S (aq) + 0,141
CuI2
-
+ e-
Cu (s) + 2 I-
+ 0,00
2 H+
+ 2 e-
H2 (g) 0
Fe3+
+ 3 e-
Fe (s) - 0,036
Pb2+
+ 2 e-
Pb (s) - 0,126
Sn2+
+ 2 e-
Sn (s) - 0,136
Ni2+
+ 2 e-
Ni (s) - 0,23
PbSO4 (s) + 2 e-
Pb (s) +
SO4
2-
- 0,356
Cd2+
+ 2 e-
Cd (s) - 0,4026
Fe2+
+ 2 e-
Fe (s) - 0,440
S(s) + 2 e-
S2-
- 0,508
Zn2+
+ 2 e-
Zn (s) - 0,7628
Al3+
+ 3 e-
Al (s) - 1,66
Ce3+
+ 3 e-
Ce (s) - 2,335
Na+
+ e-
Na (Hg) (l) - 2,711
Li+
+ e-
Li (Hg) (l) - 3,045
Conversion factors
Force (= mass x acceleration)
1 N = 1 kg m/s²
1 dyn = 10-5
N
1 kp = 9,806 N
Pressure (= force / area)
1 Pa = 1 N/m² = 10-5
bar
101325 Pa = 1 atm = 760 Torr
760 mmHg = 10,33 mWS
1 psi = 6 895 Pa
Energy (= force x distance; el. work
= charge x voltage)
1 J = 1 N m = 1 m³ Pa
1 erg = 10-7
J
1 eV = 1,602110-19
J
1 eV = 96 494 J/mol
1 cal = 4,184 J
Power (= work / time)
1 W = 1 J/s
1 kWh = 3600000 J/s
1 hp = 745,7 W](https://image.slidesharecdn.com/physicalchemistryformulary-130809201508-phpapp02/85/Physical-chemistry-formulary-the-laws-of-thermodynamics-hold-36-320.jpg)

