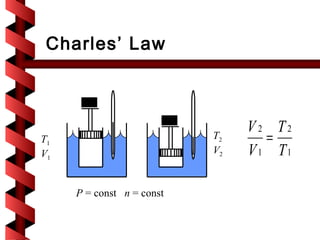
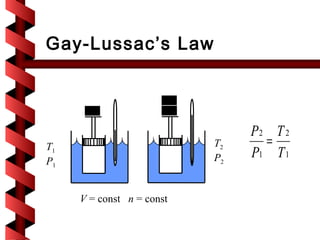
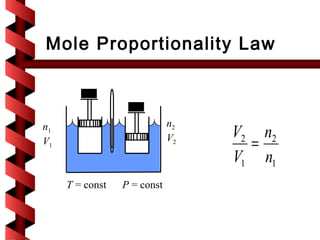
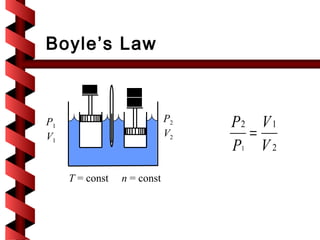
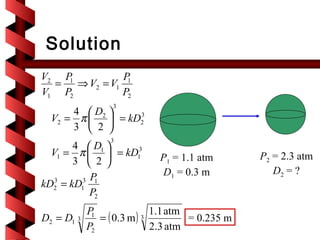
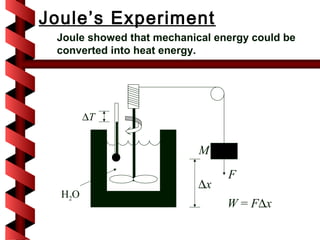
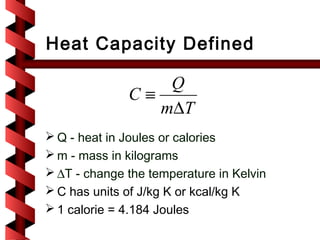
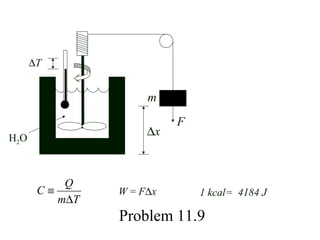
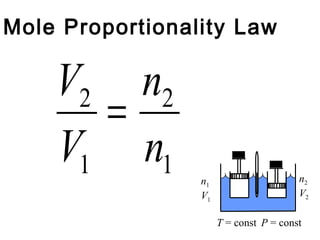
Here are the key steps to solve this problem:
1) Mass of coke = Volume x Density = 335 ml x 1 g/ml = 335 g
2) Temperature change = Room temperature (assume 70°F) - Coke temperature (35°F) = 70°F - 35°F = 35°F
3) Heat required to change temperature of coke = Mass x Heat capacity x Temperature change
= 335 g x 1 cal/g°C x 35°F/°C
= 335 x 1 x 35 = 11,725 cal
4) Convert calories to Joules: 11,725 cal x 4184 J/cal = 49,000 J
So the number