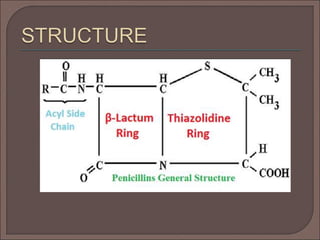
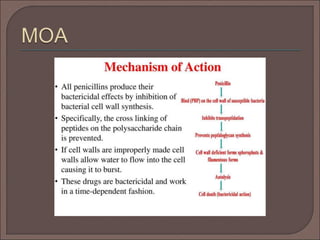
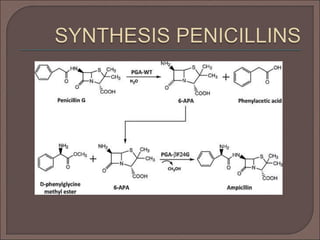
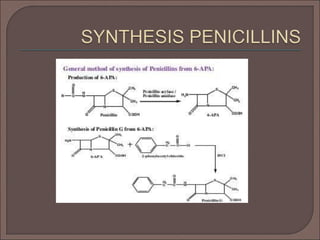
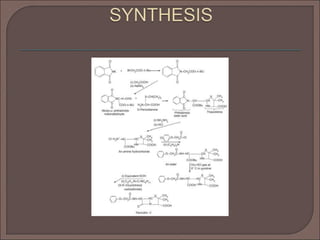
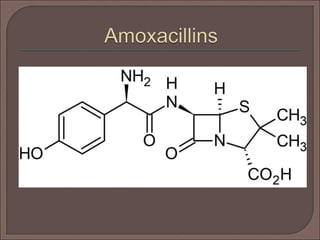
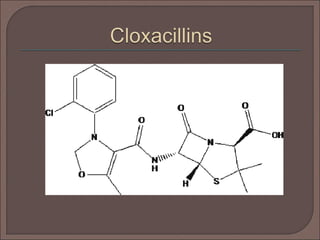
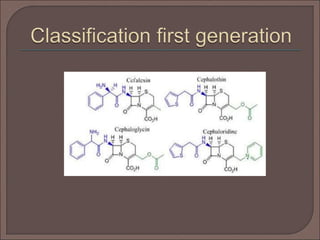
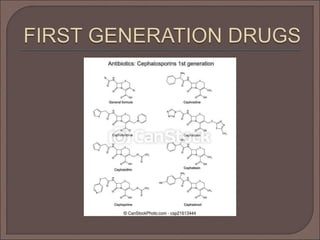
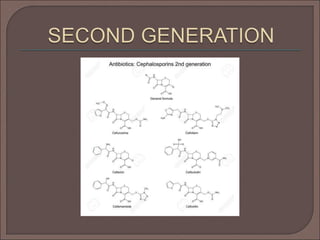
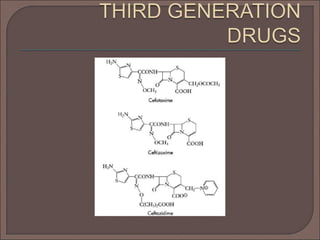
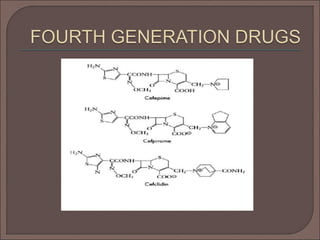
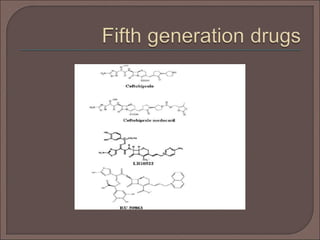
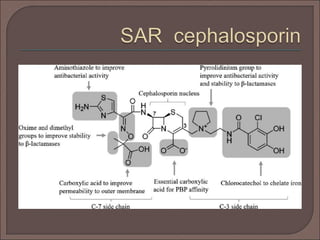
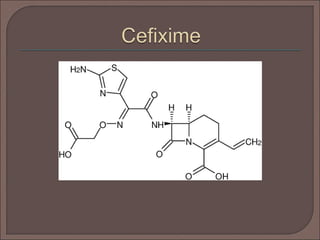
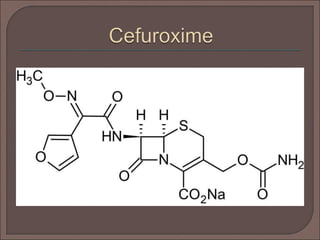
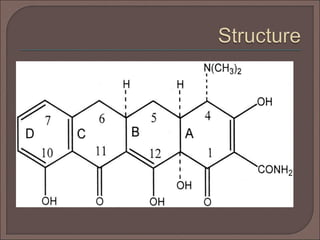
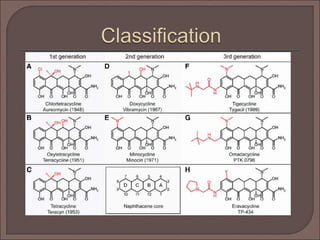
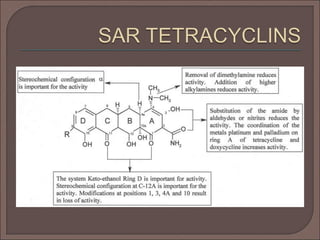
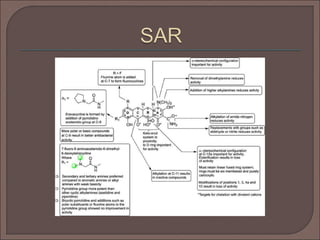
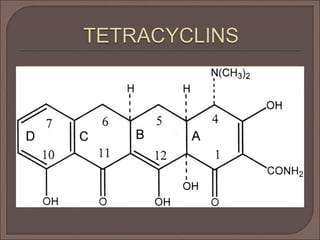
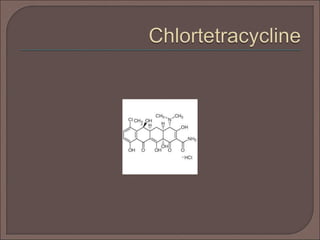
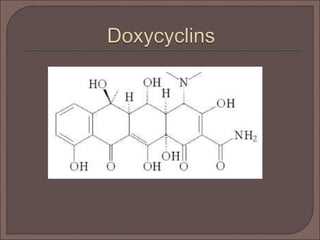
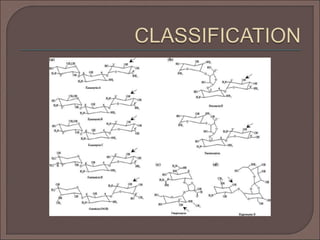
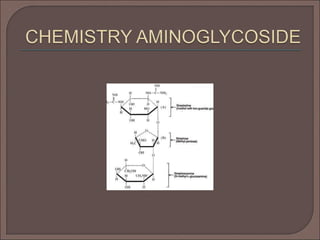
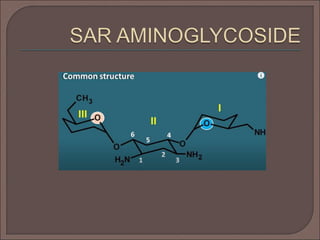
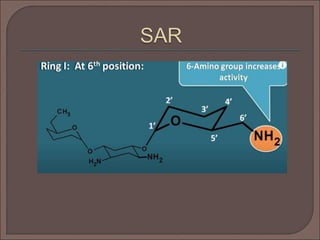
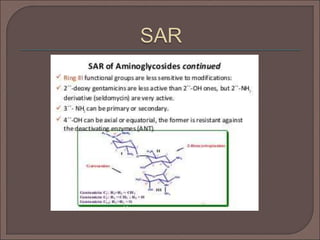
The document provides an overview of antibiotics, focusing on their use as antibacterial agents, particularly penicillins and cephalosporins, detailing their mechanisms of action, properties, and applications in treating bacterial infections. It explains various chemical structures and modifications that enhance antibiotic activity and resistance to degradation. Additionally, it covers classifications, side effects, and specific examples of antibiotics along with their therapeutic uses.