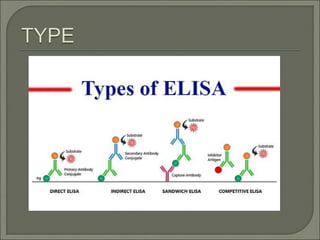
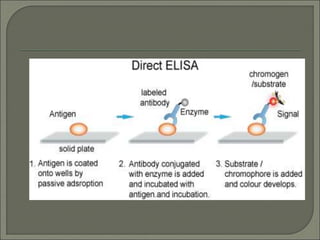
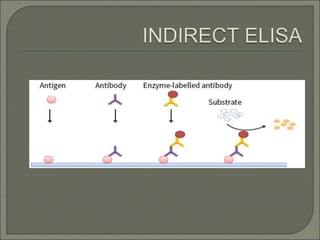
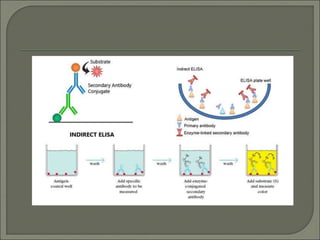
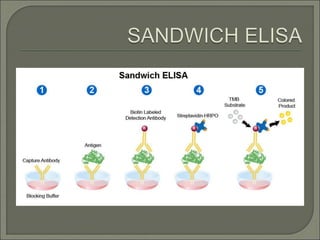
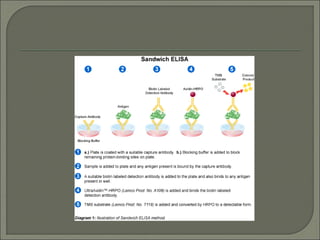
The document describes various types of ELISA (enzyme-linked immunosorbent assay) tests, including direct ELISA, indirect ELISA, sandwich ELISA, and competitive ELISA. It explains the basic principles and procedures for each type. ELISA tests use enzyme and antibody or antigen reactions to detect substances like proteins, hormones, antibodies, or drugs in samples. The tests are used for medical diagnostic purposes like detecting infections and allergies.