The Universal Immunization Programme (UIP) was launched by the Government of India in 1985 to reduce mortality and morbidity from six vaccine preventable diseases (VPDs) and has evolved into a crucial part of the National Rural Health Mission since 2005. It provides routine immunizations, organizes campaigns, and addresses vaccine logistics while ensuring safety and efficiency through a cold chain system. Major achievements include the eradication of smallpox and a three-year polio-free status in India.




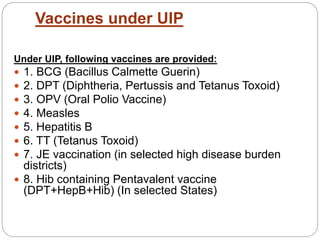






![ The diseases being targeted are diphtheria,
whooping cough, tetanus, poliomyelitis,
tuberculosis, measles and Hepatitis B. In addition
to these, vaccines for Japanese Encephalitis[2]
and Haemophilus influenzae type B are also
being provided in selected states.](https://image.slidesharecdn.com/universalimmunisationprogram-171120044520/85/Universal-immunisation-program-17-320.jpg)




