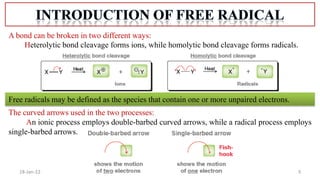
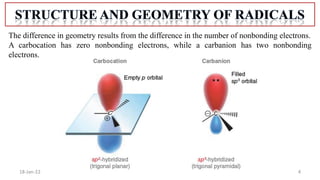
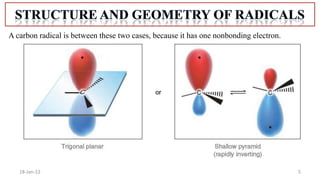
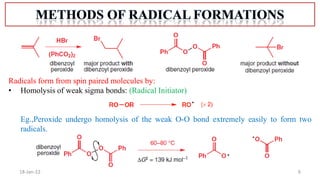
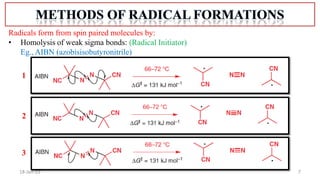
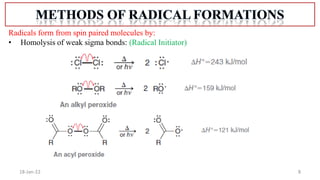
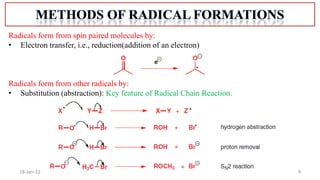
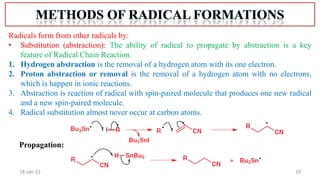
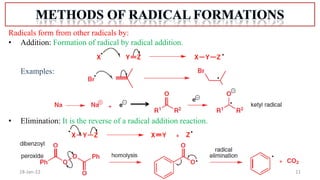
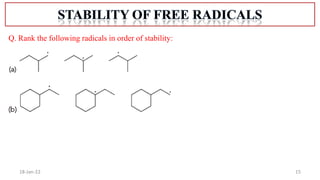
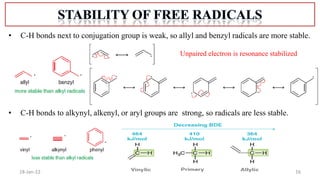
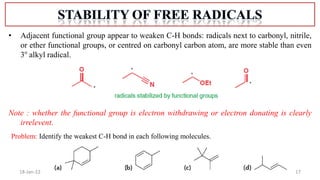
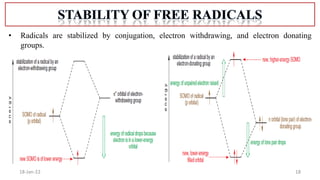
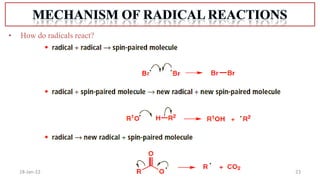
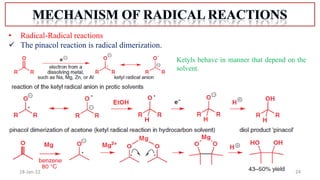
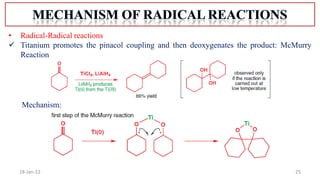
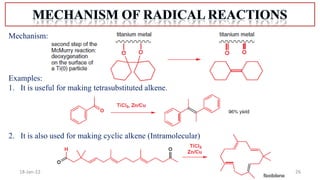
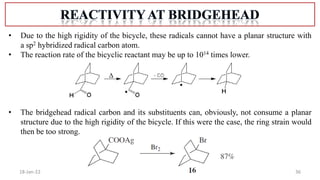
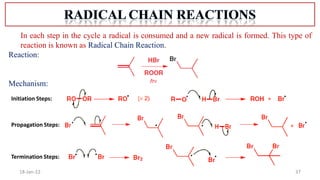
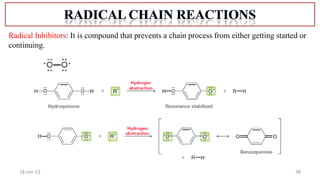
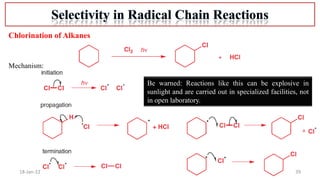
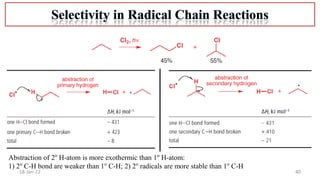
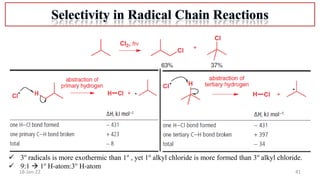
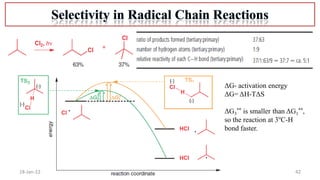
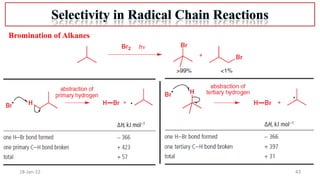
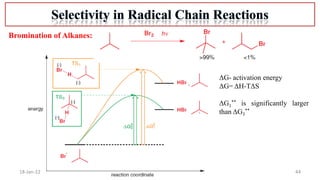
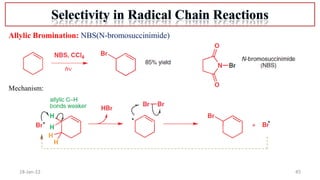
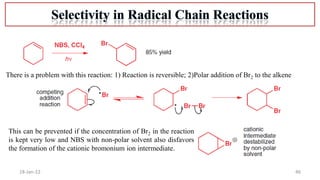
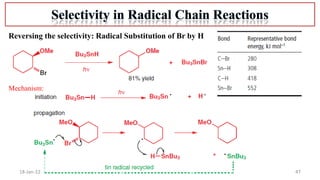
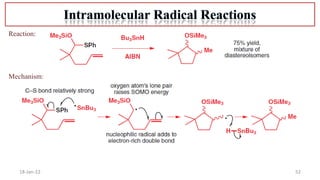
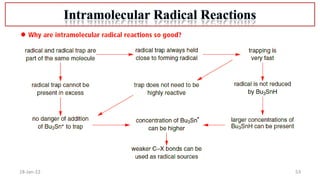
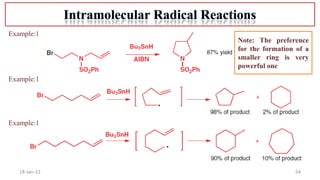
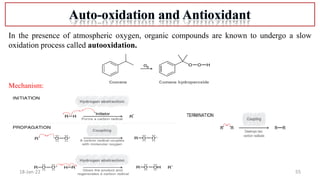
The document summarizes a guest lecture on free radicals that covered several topics:
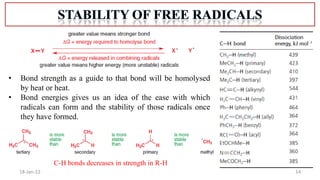
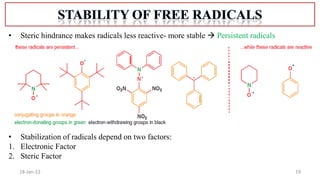
1. The structure and formation of free radicals through homolytic bond cleavage and their stability based on factors like conjugation and sterics.
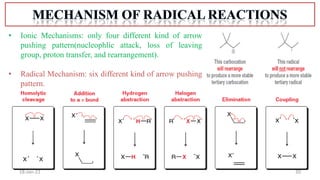
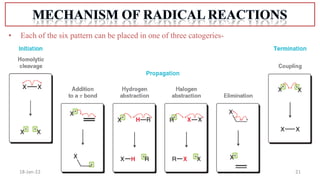
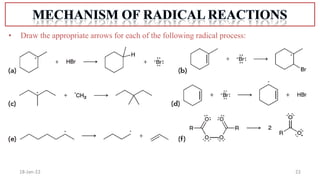
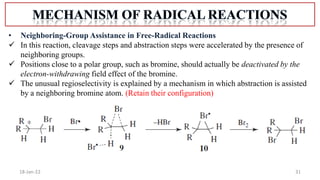
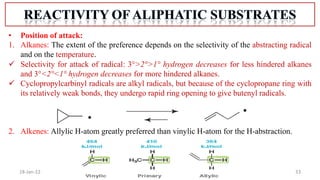
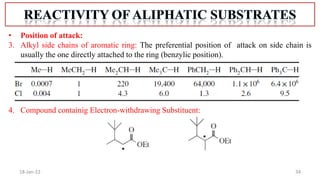
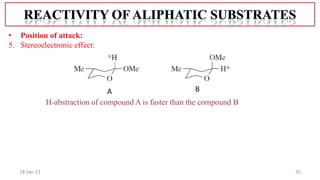
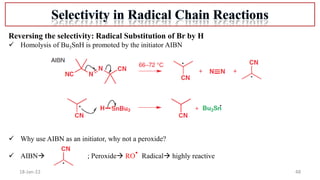
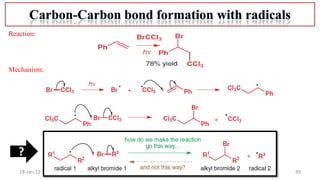
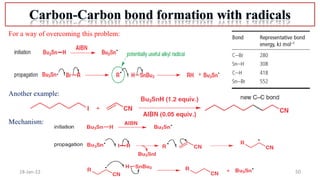
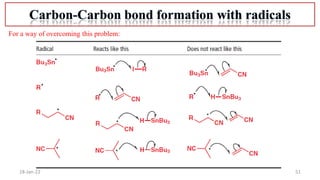
2. Mechanisms of radical substitution reactions including neighboring group assistance and reactivity based on position.
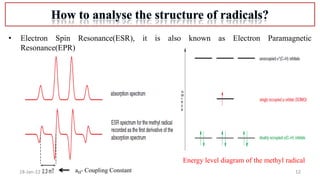
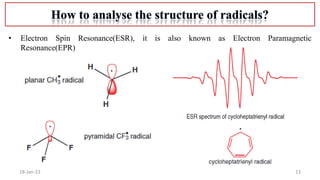
3. Methods to characterize radicals using electron spin resonance spectroscopy.
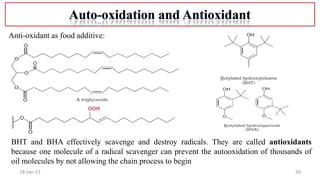
4. Examples of radical reactions including halogenation, allylic substitution, and autooxidation.