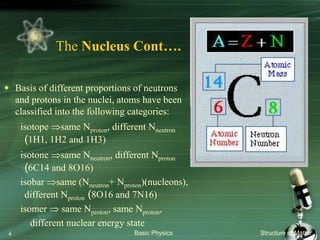
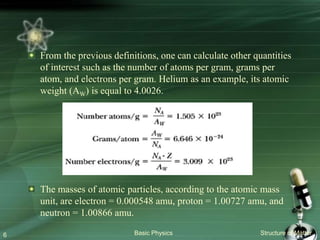
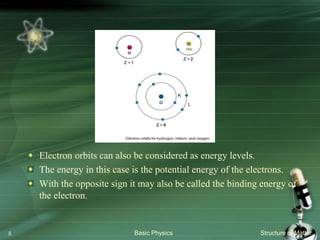
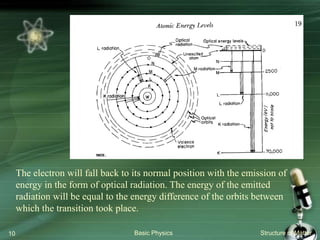
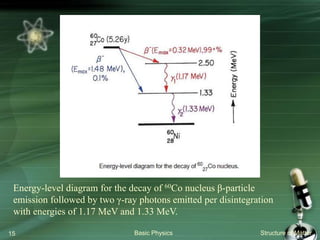
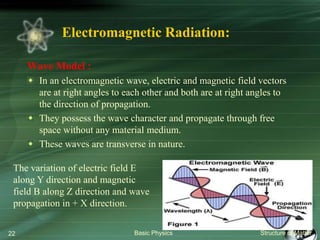
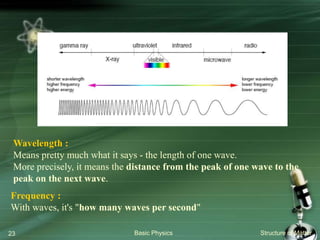
The document summarizes the structure of the atom. It discusses that atoms are composed of a nucleus containing protons and neutrons, surrounded by electrons in orbits. The nucleus is much smaller than the atom but contains most of its mass. Properties of atoms are determined by the number and arrangement of protons, neutrons, and electrons. Electrons can occupy different energy levels in orbits around the nucleus. Nuclear forces hold the nucleus together, while electromagnetic forces between protons cause repulsion.