Embed presentation
Download to read offline

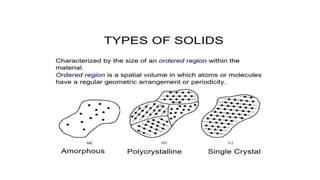
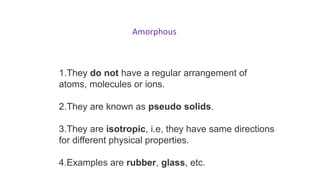



There are three main types of solids: amorphous solids which have an irregular atomic structure and are isotropic, polycrystalline solids which are aggregates of many small crystals joined at grain boundaries, and single crystals which consist of a single crystal with no grain boundaries.




