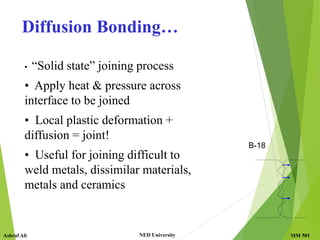
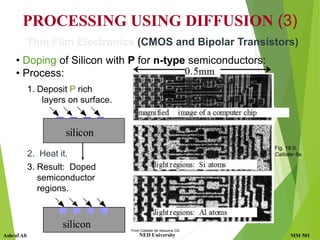
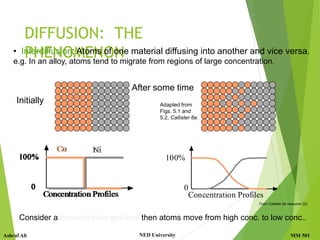
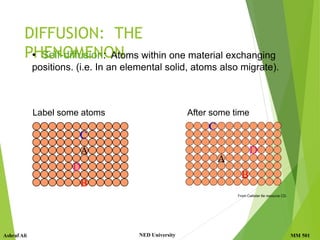
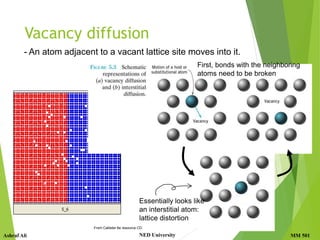
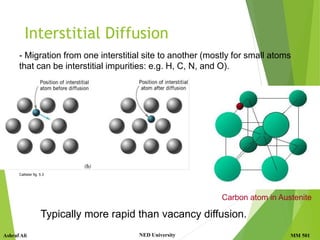
This document discusses diffusion in solids, which is the phenomenon of material transport through atomic motion even in crystalline solids. Diffusion plays an important role in materials processing and phase transformations. It occurs through mechanisms like vacancy diffusion or interstitial diffusion. Fick's laws of diffusion can be used to model and predict diffusion rates based on factors like concentration gradients, diffusion coefficients, and temperature. Applications of diffusion include alloying, case hardening, doping of semiconductors, corrosion protection, and diffusion bonding.









![MM 501
Ashraf Ali NED University
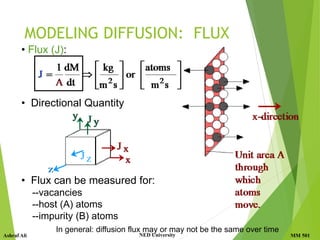
MODELING DIFFUSION
What causes net flow of atoms?
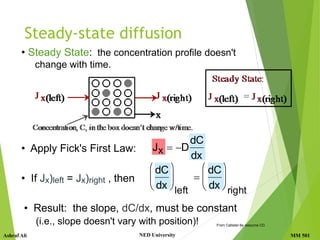
• Concentration Profile, C(x): [kg/m3]
• Fick's First Law:
Concentration
of Cu [kg/m 3]
Concentration
of Ni [kg/m 3]
Position, x
Cu flux Ni flux
• The steeper the concentration profile,
the greater the flux!
Adapted from
Fig. 5.2(c),
Callister 6e.](https://image.slidesharecdn.com/diffusioninsolids-lecture-1-220912150542-a40c90f6/85/Diffusion-in-Solids-Lecture-1-ppt-20-320.jpg)