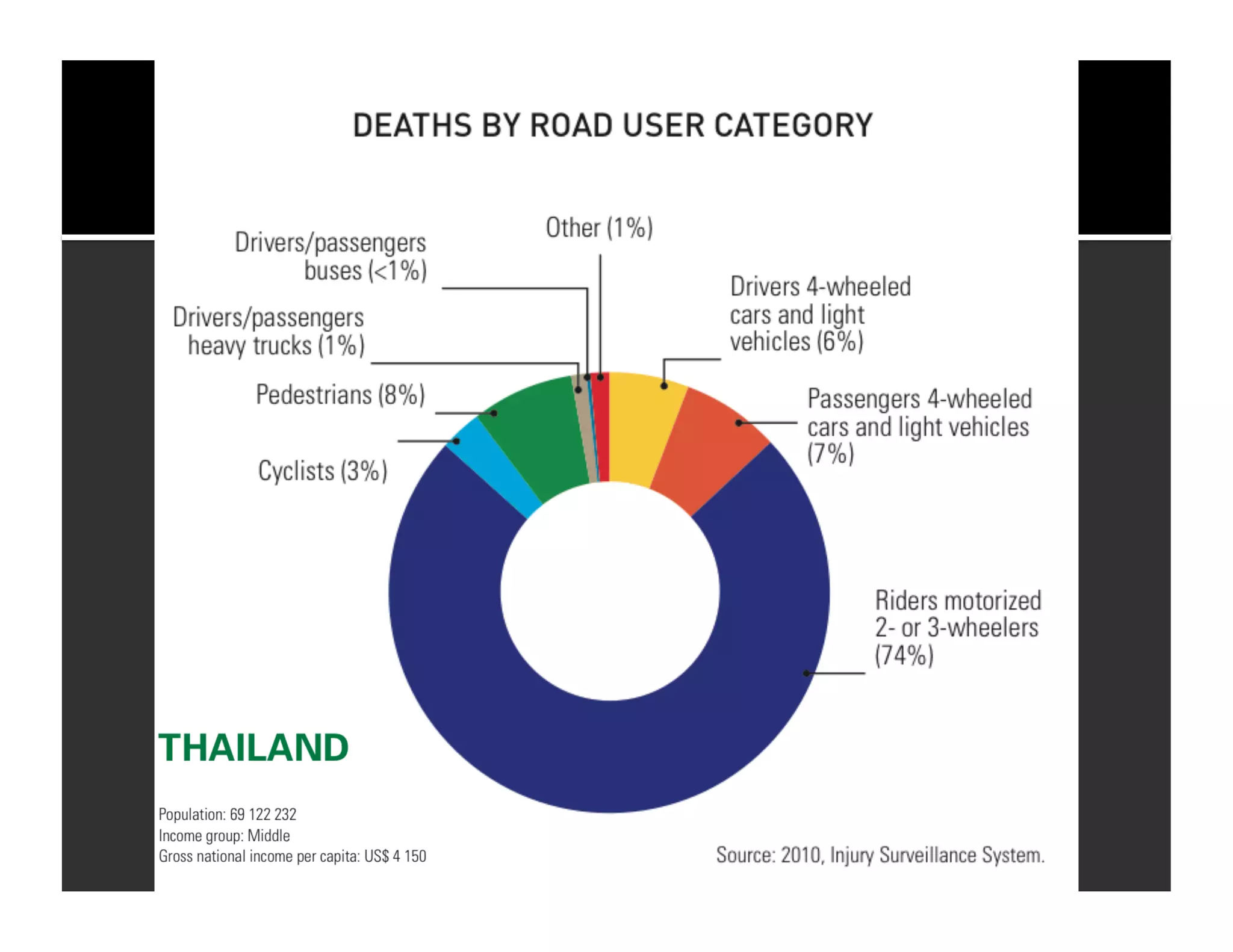
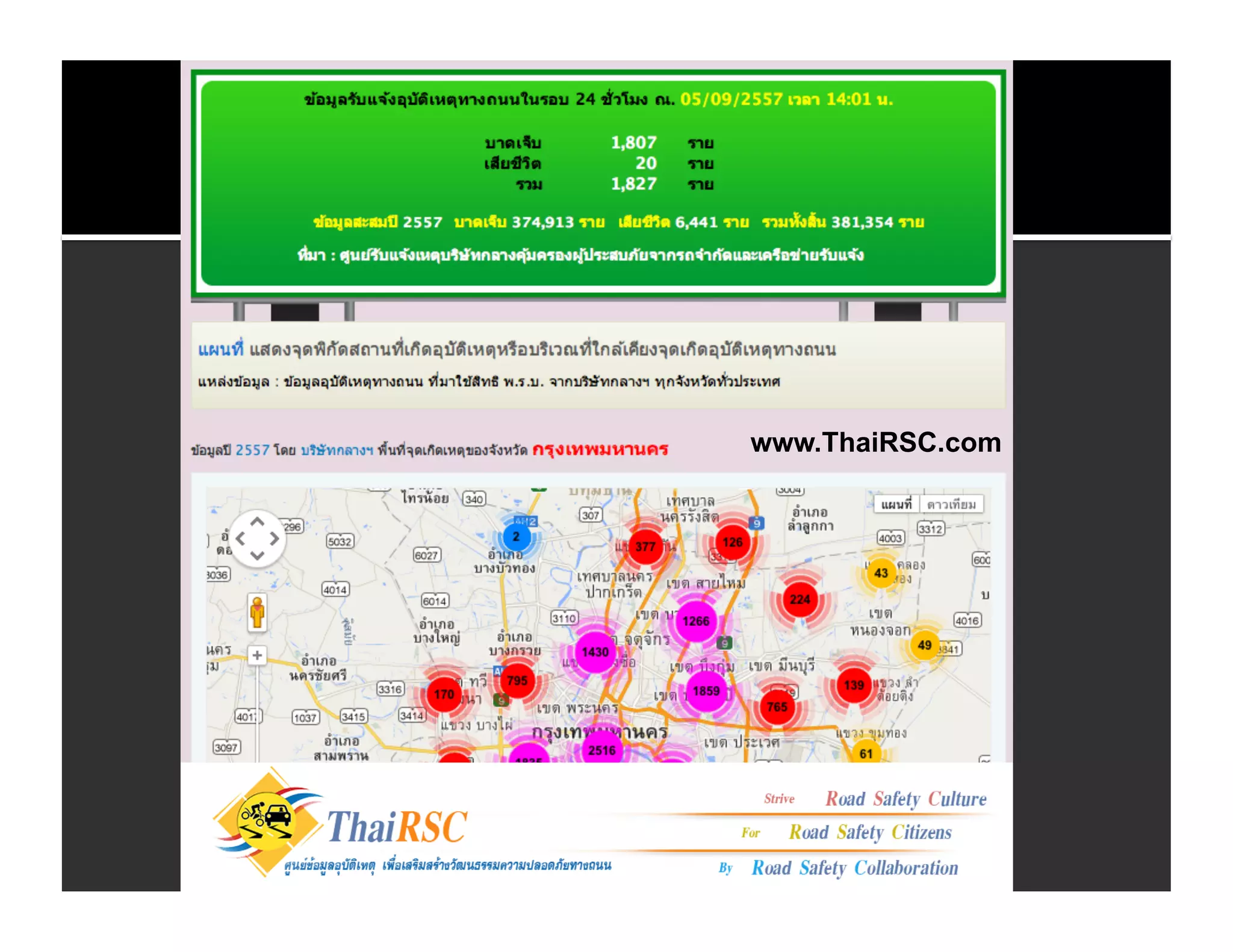
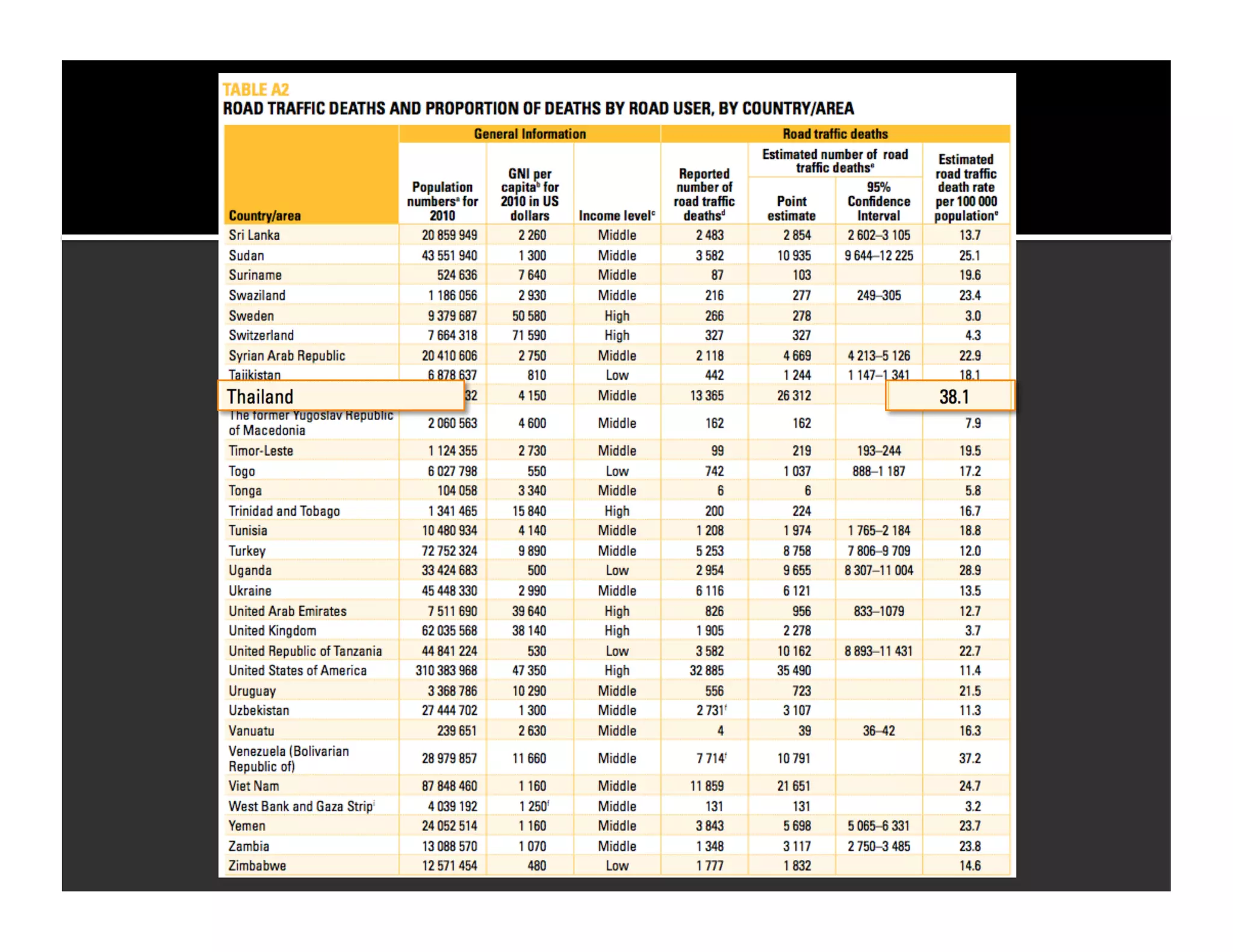
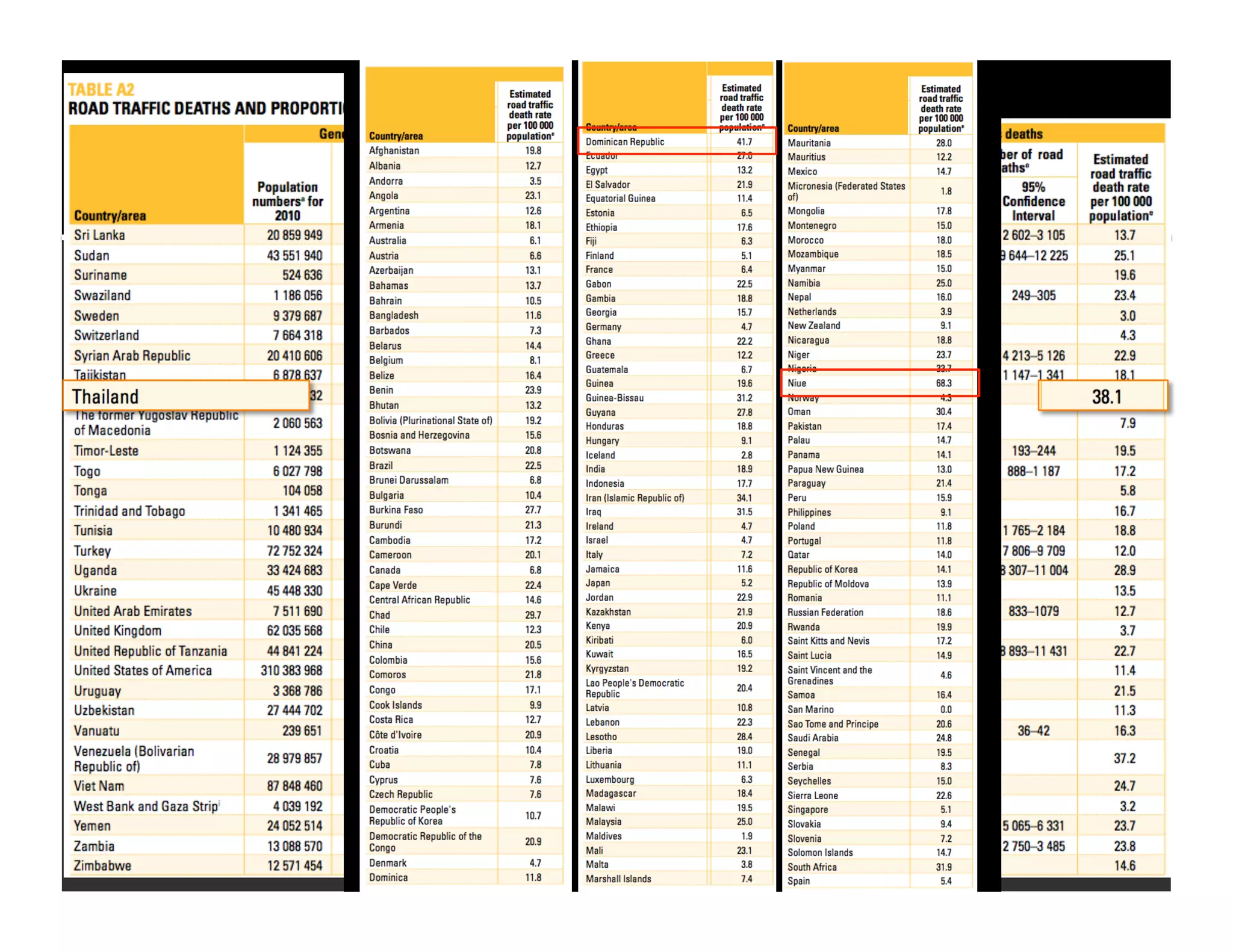
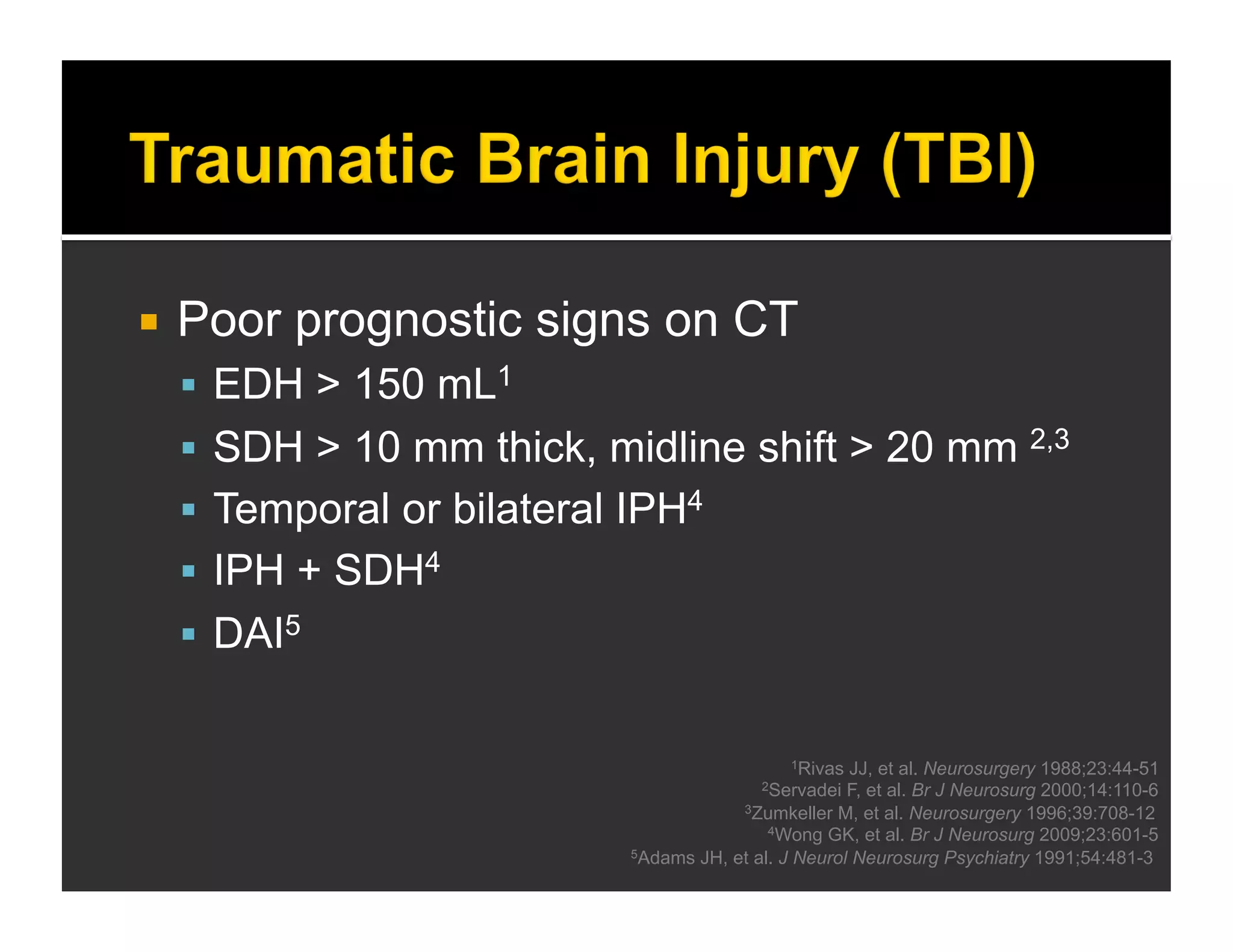
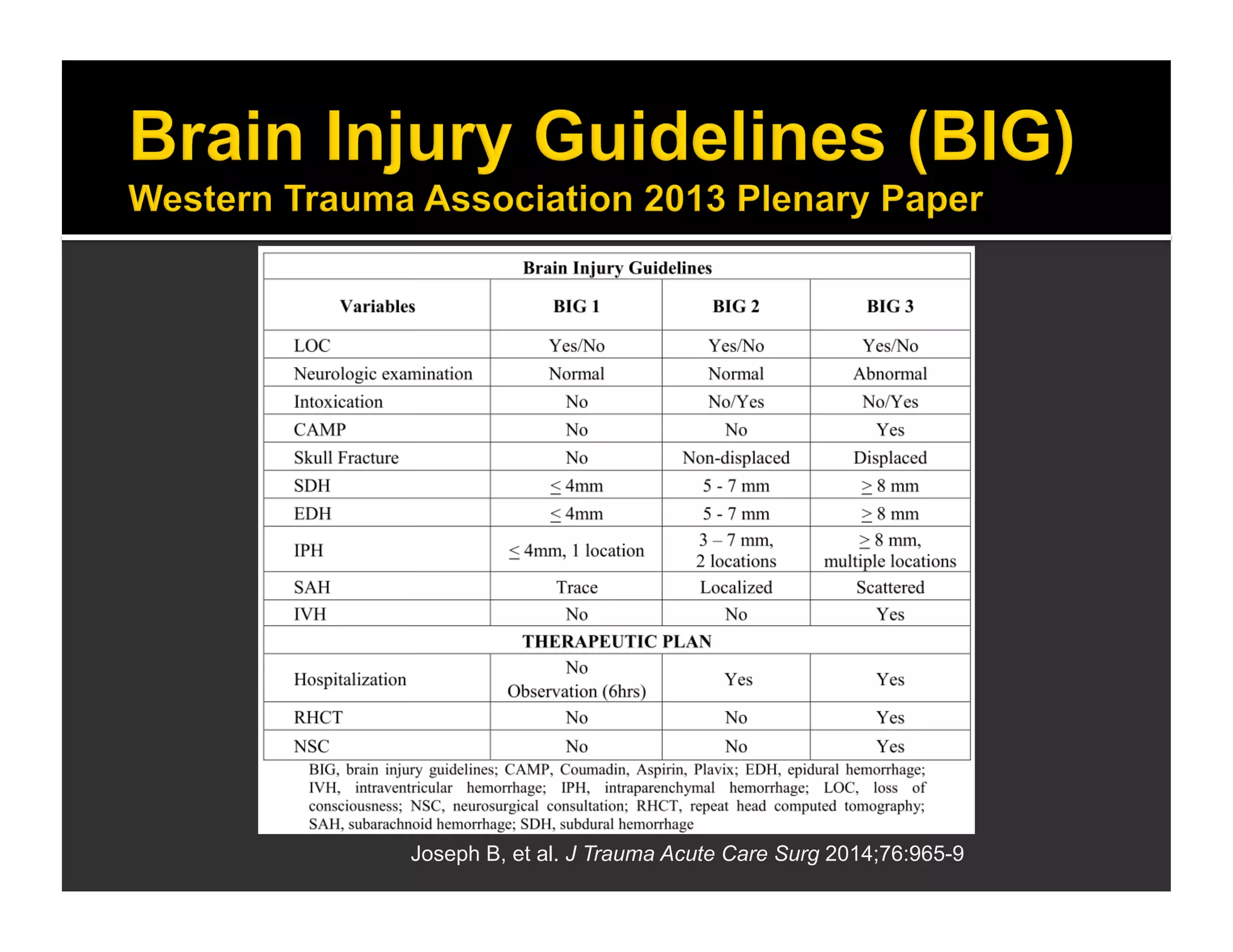
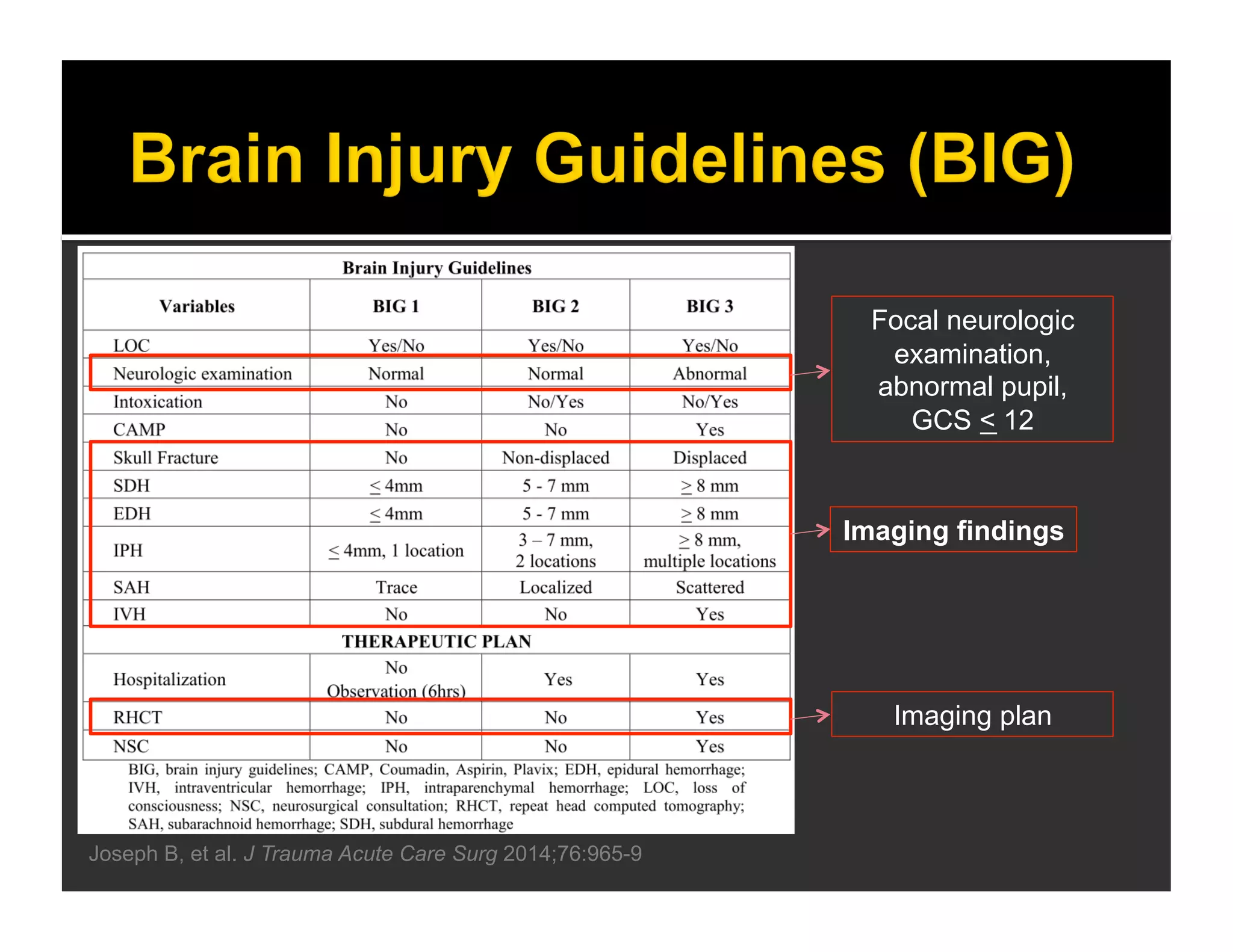
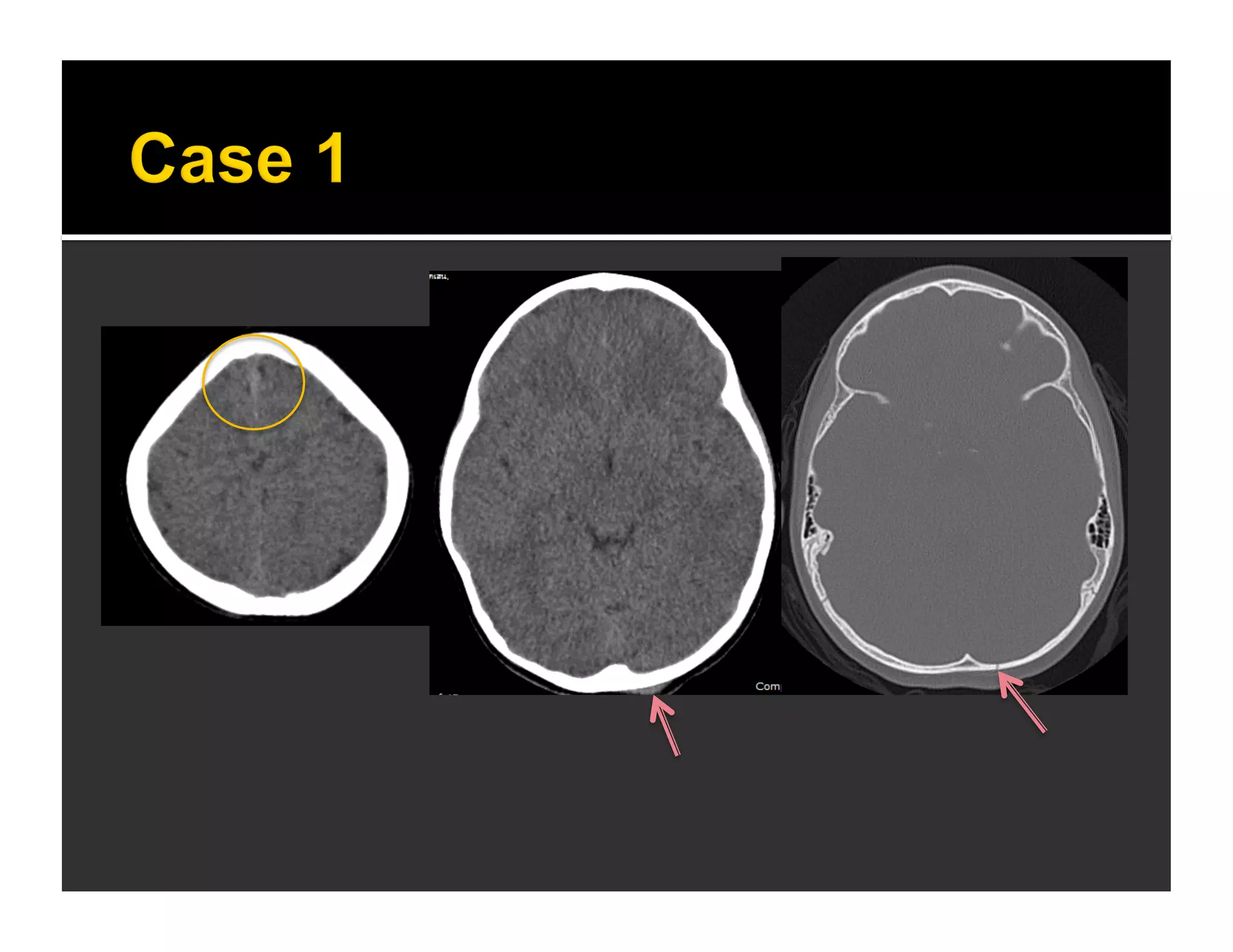
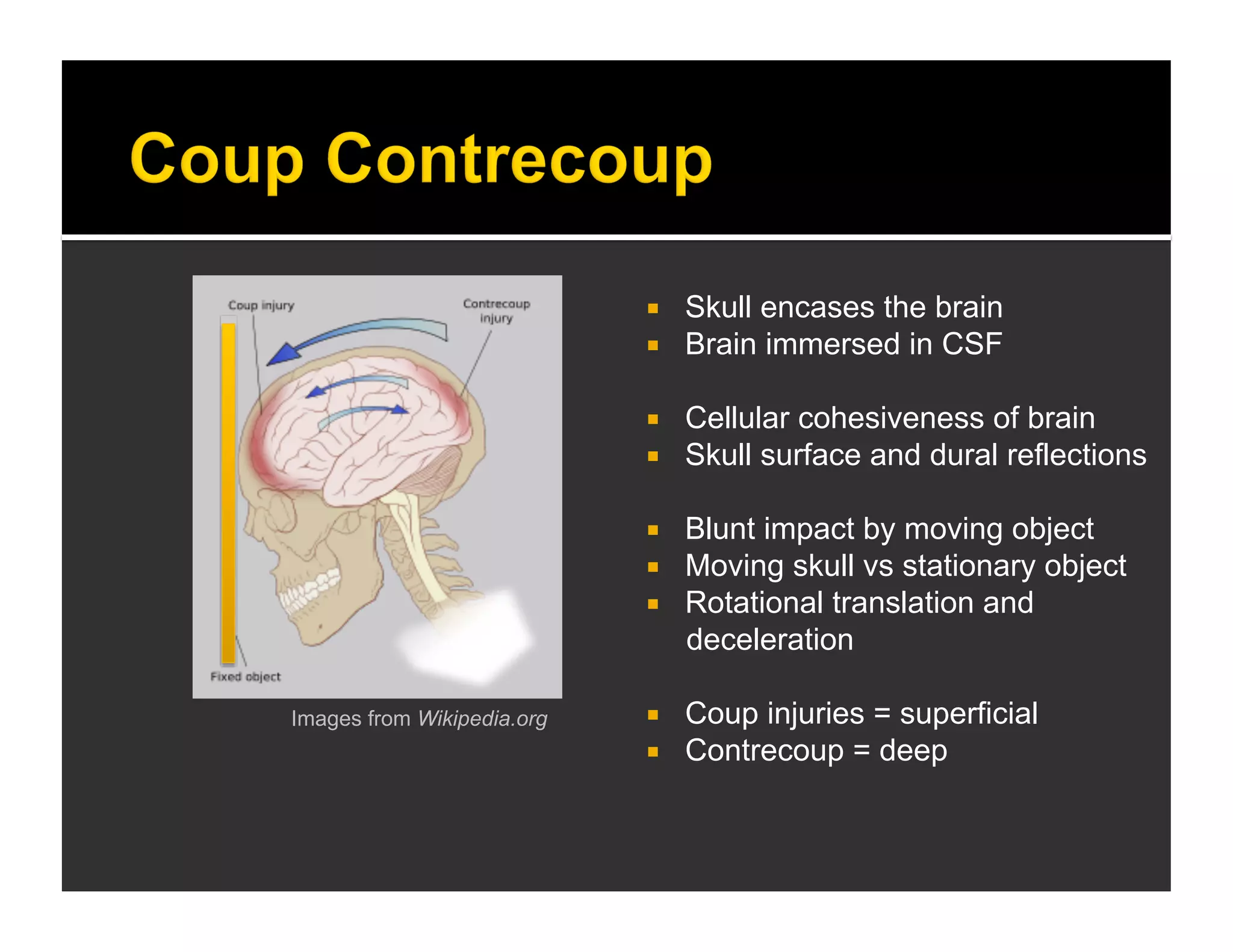
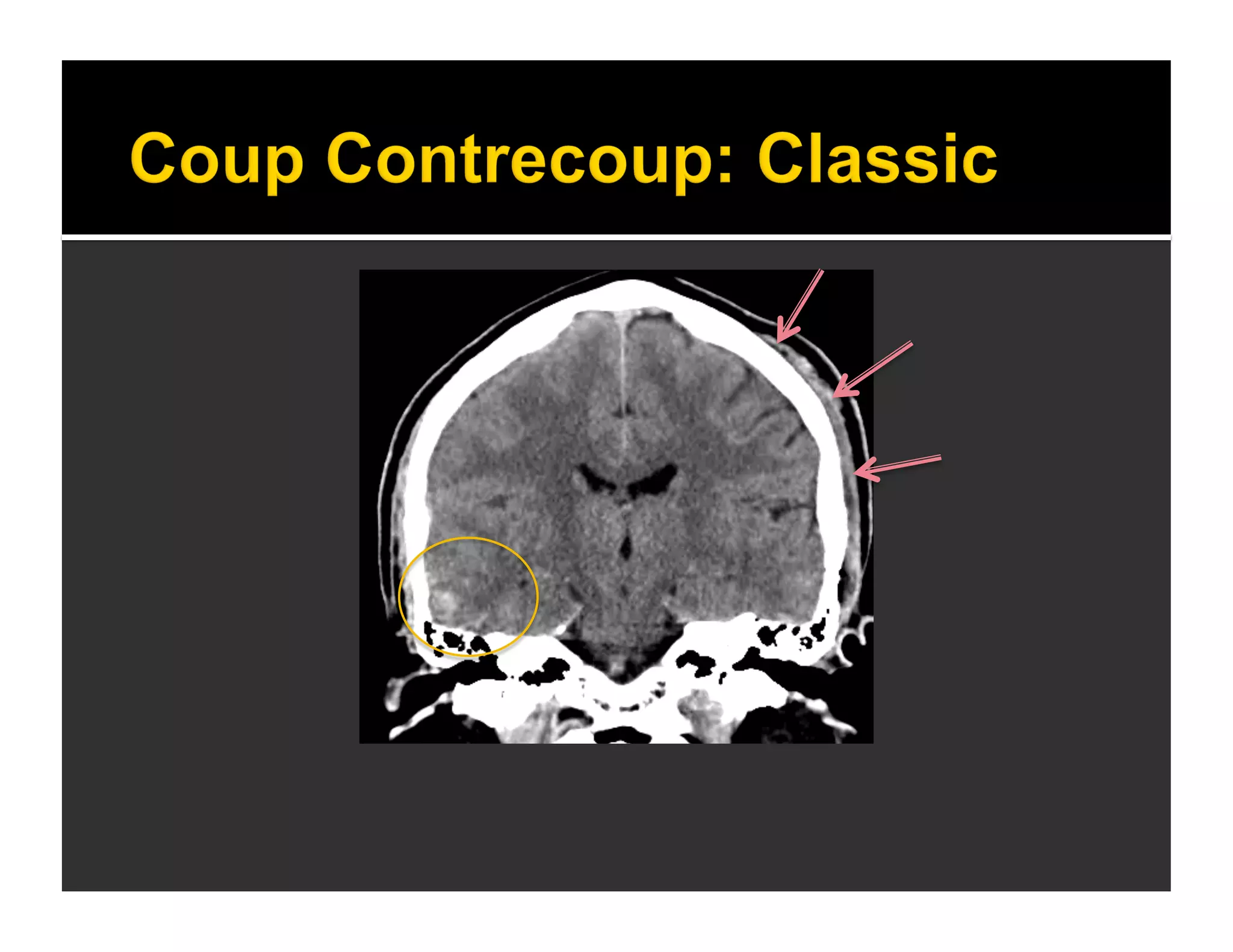
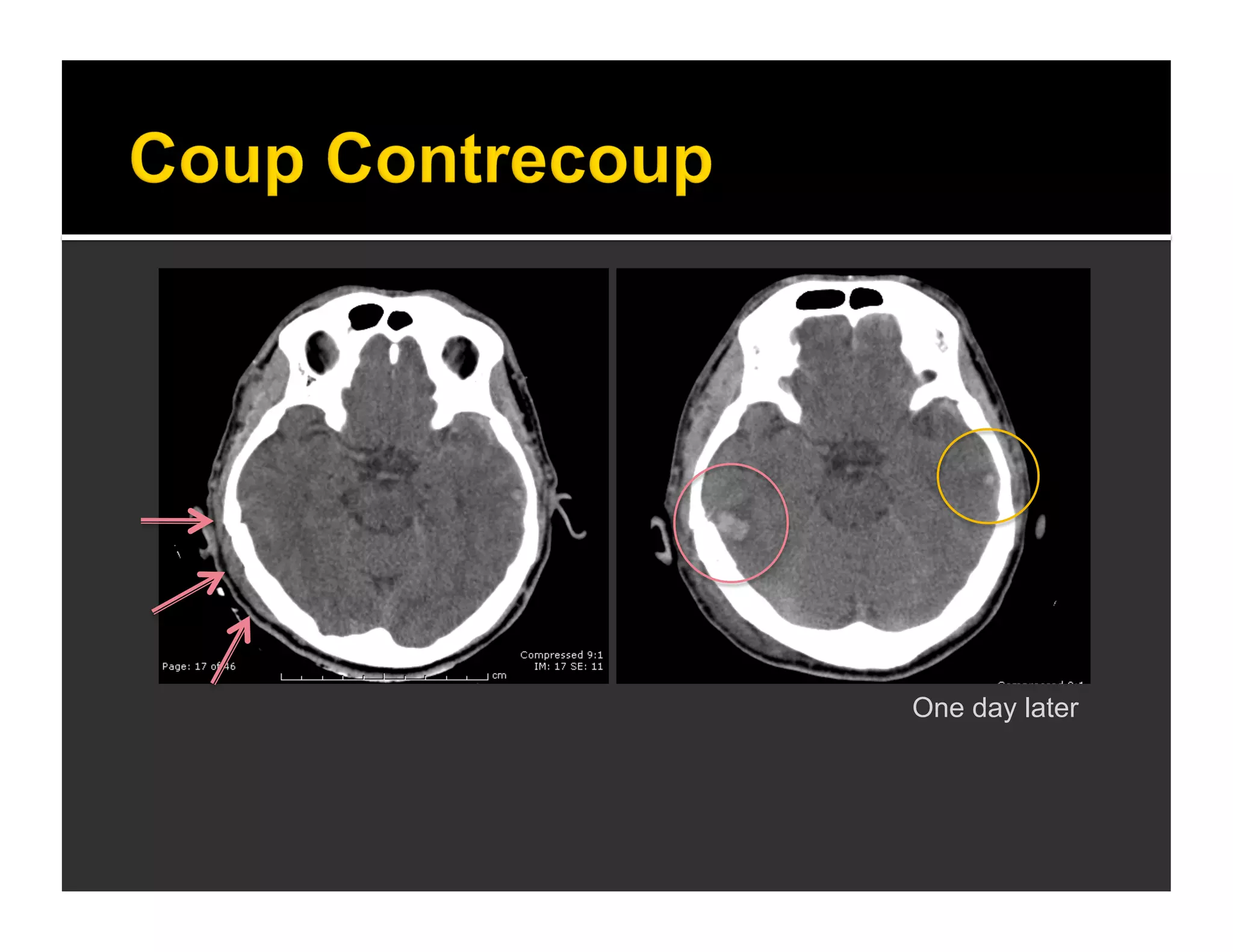
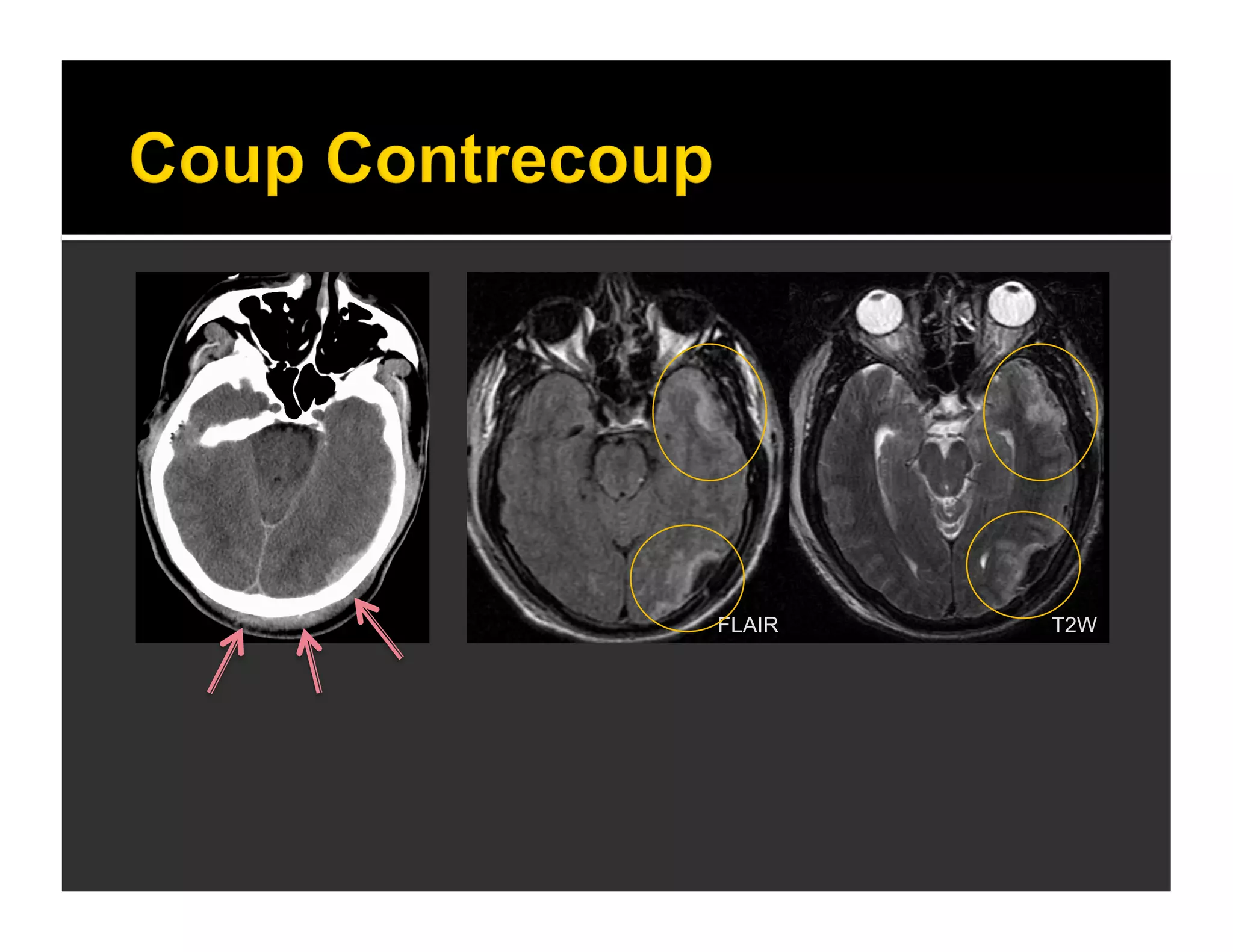
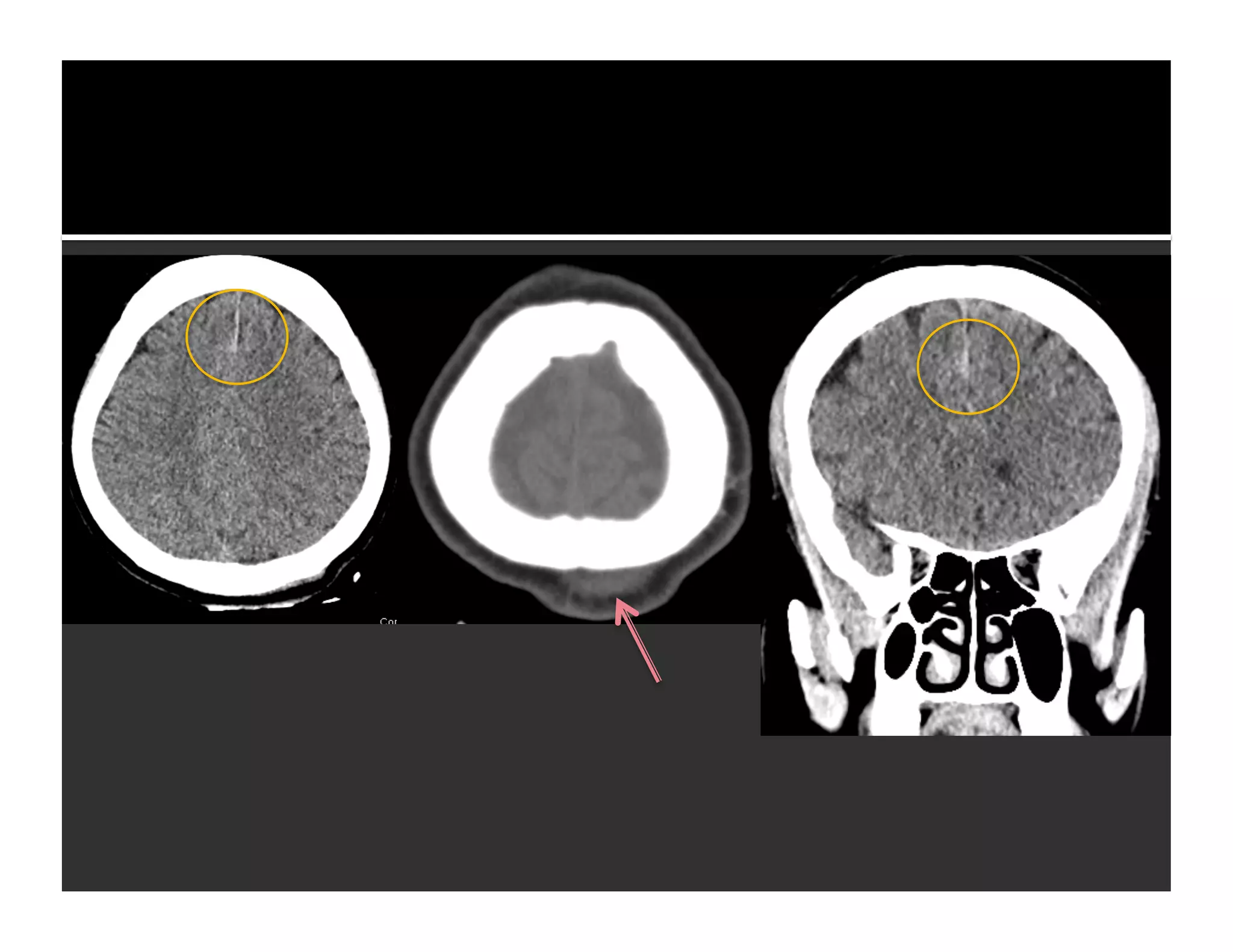
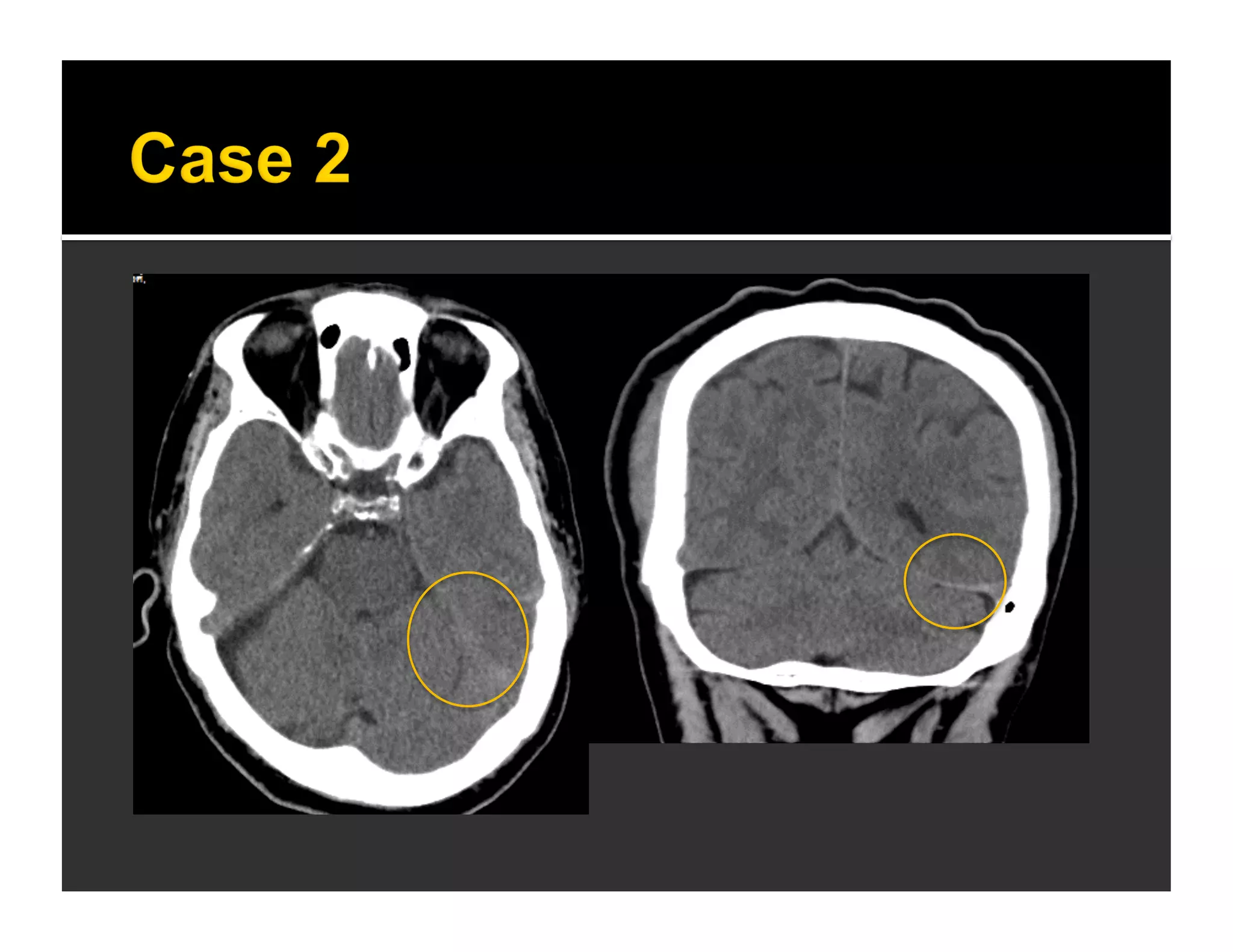
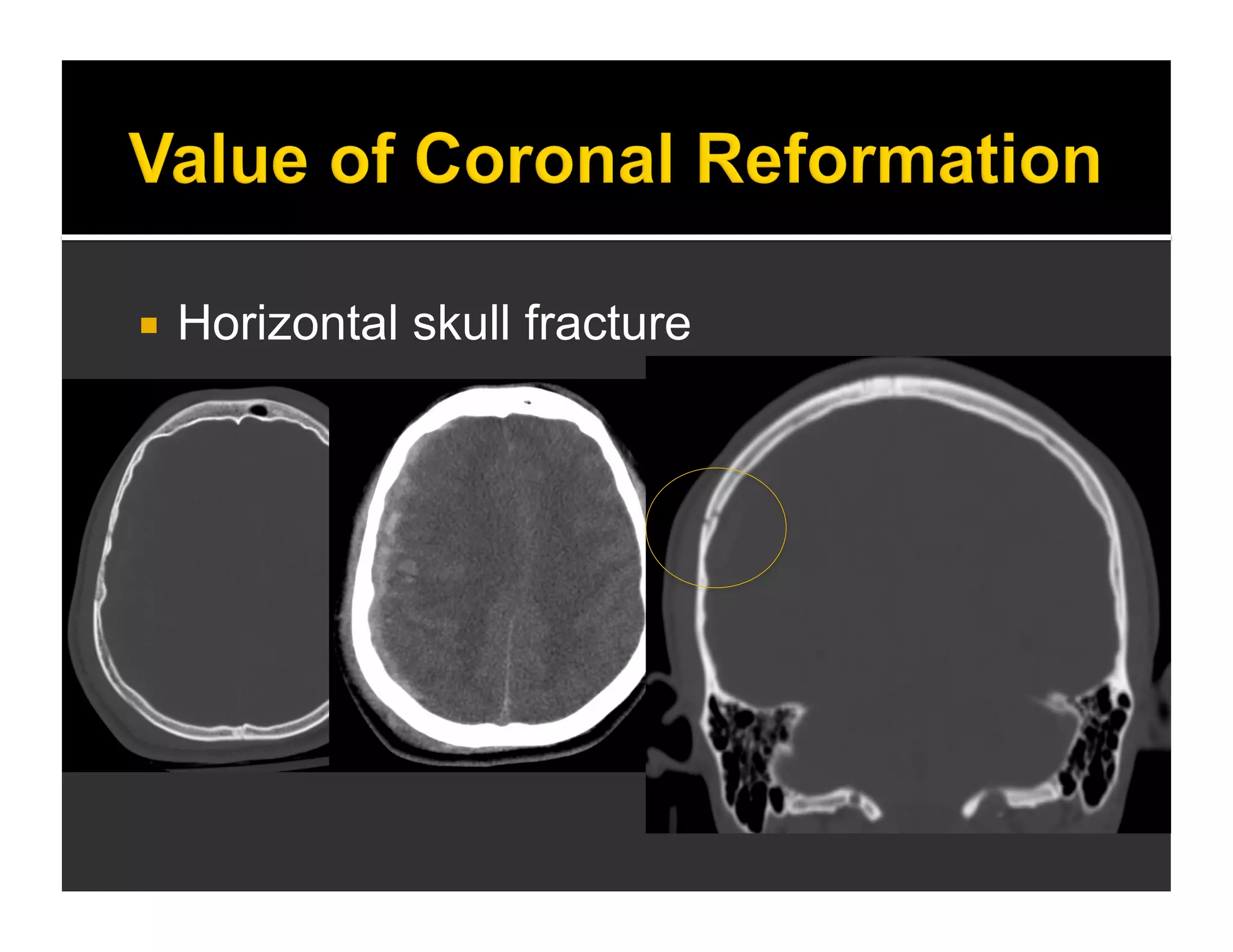
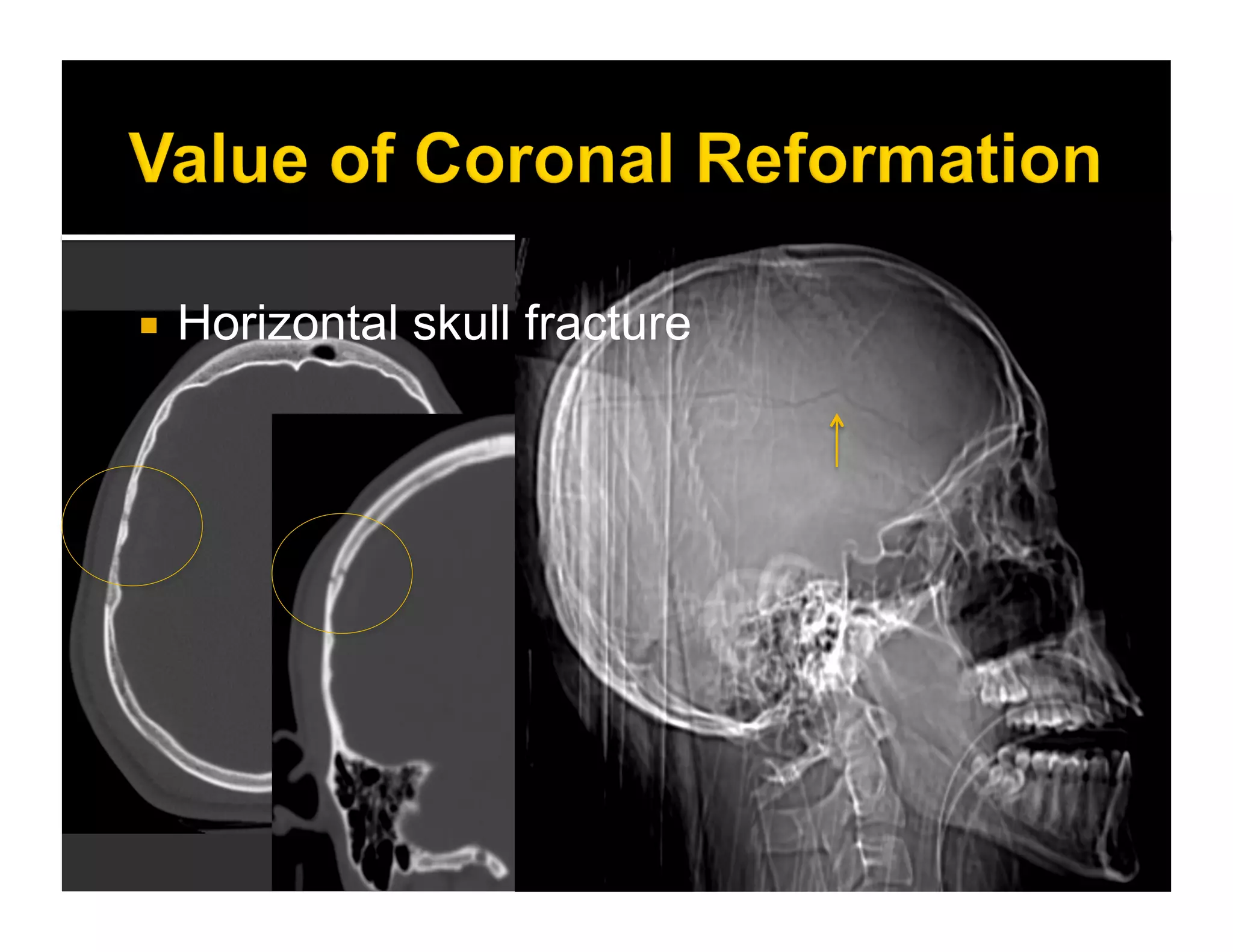
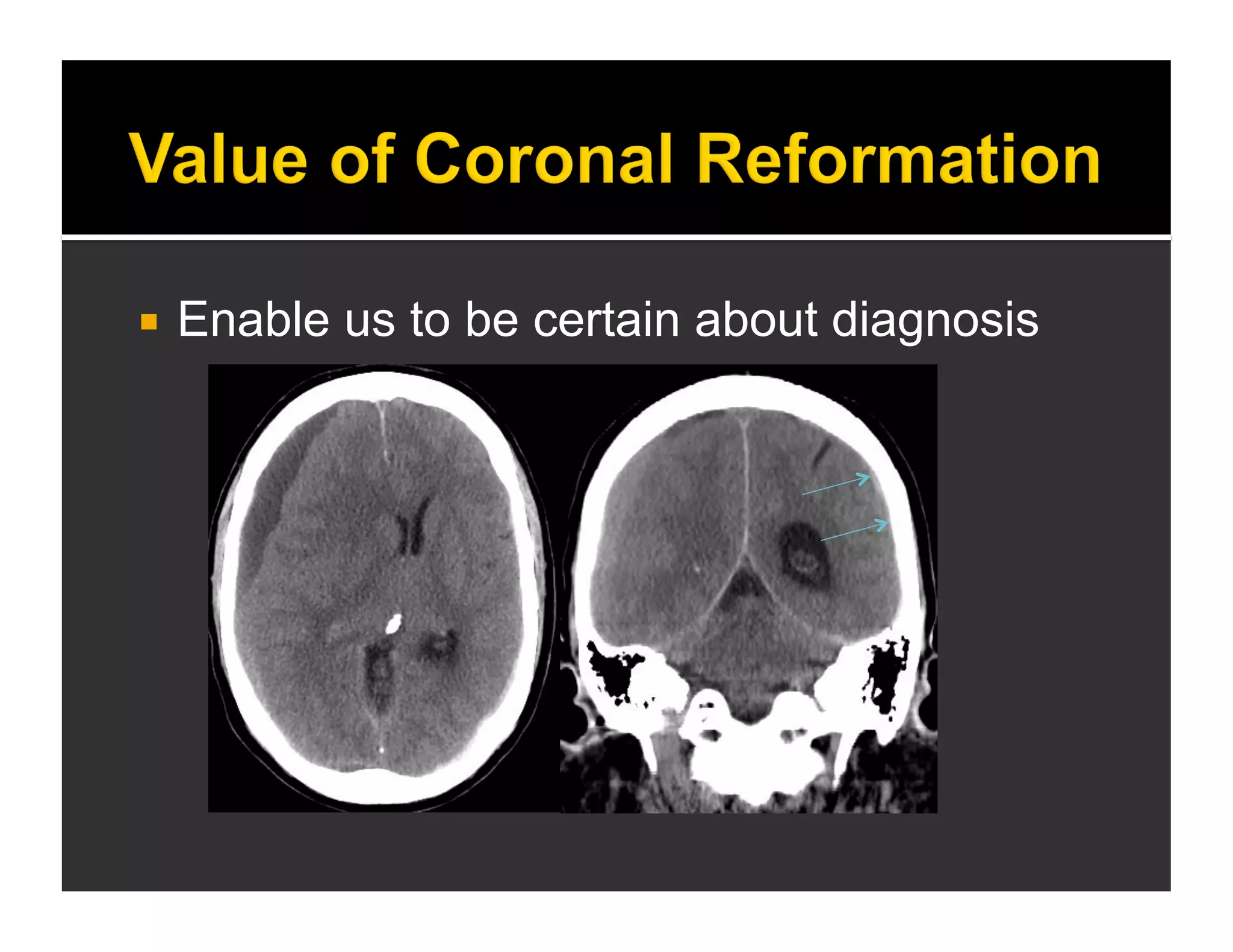
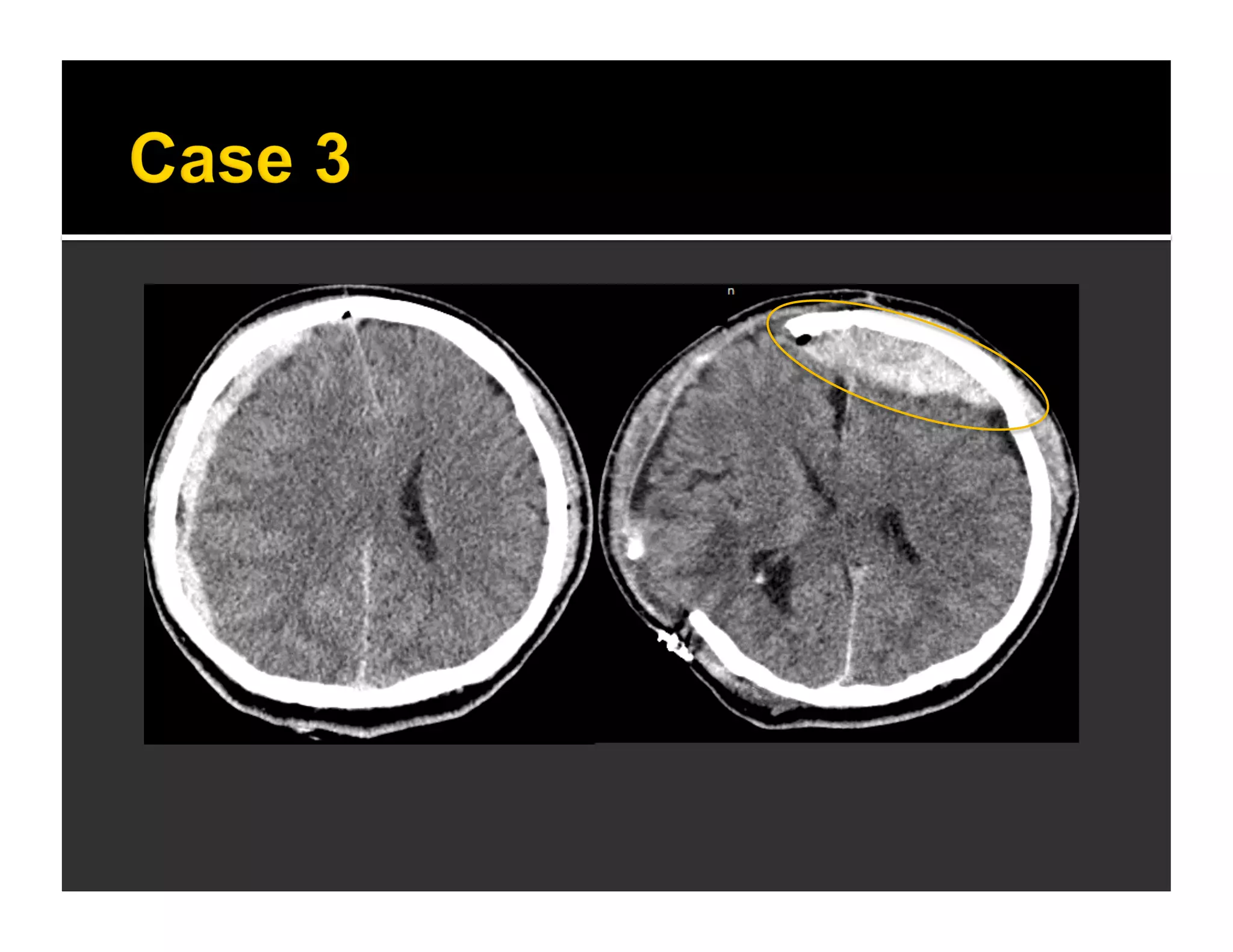
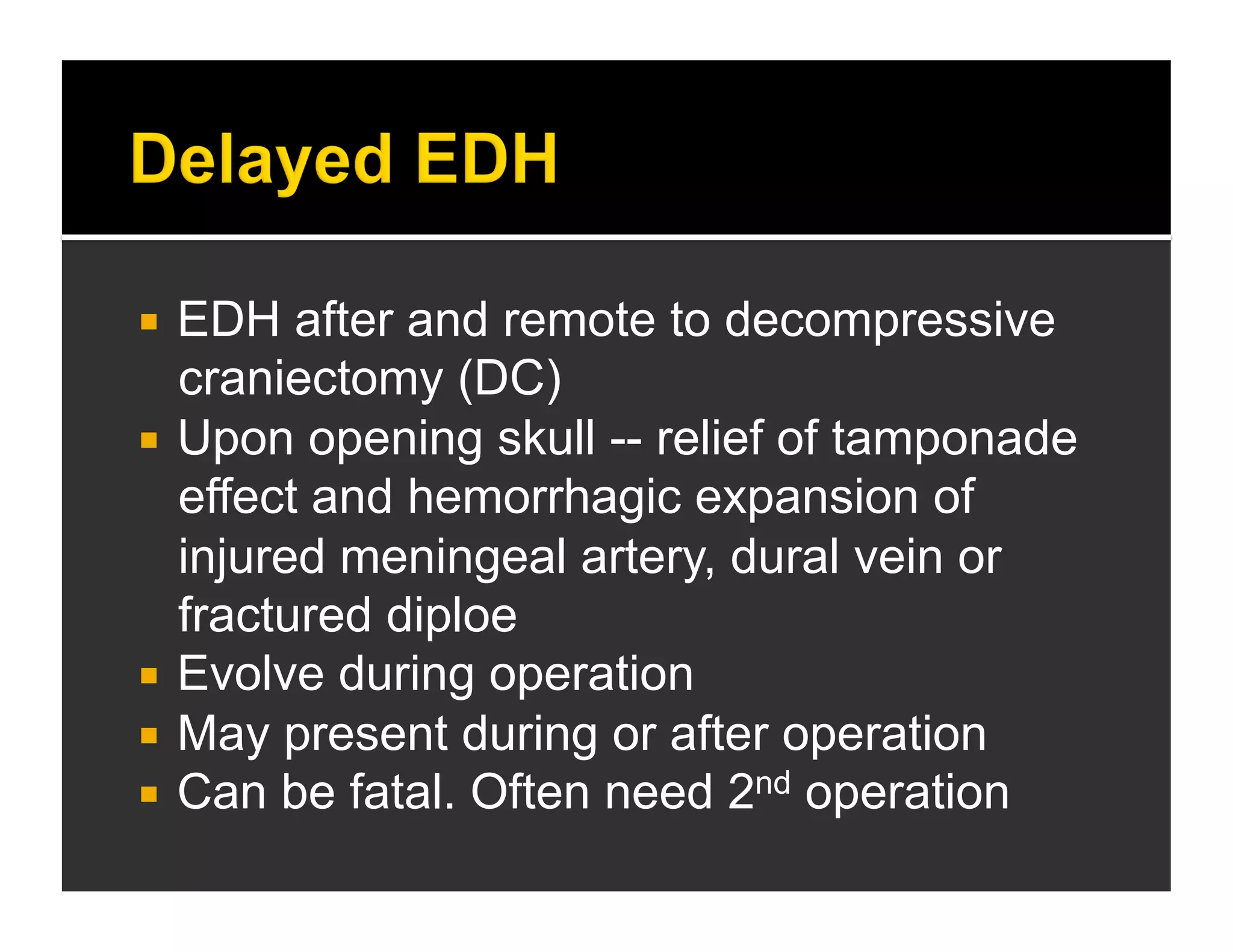
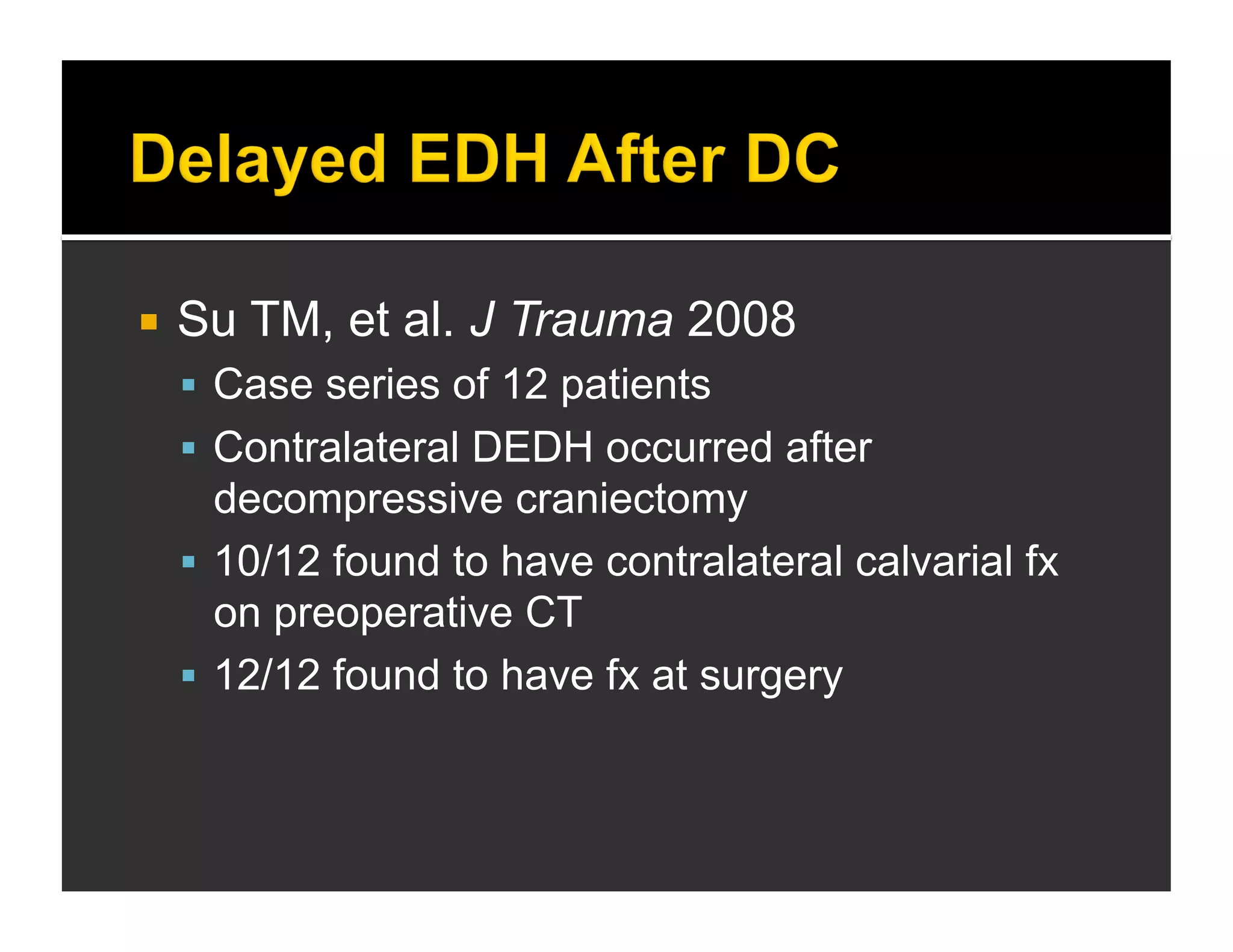
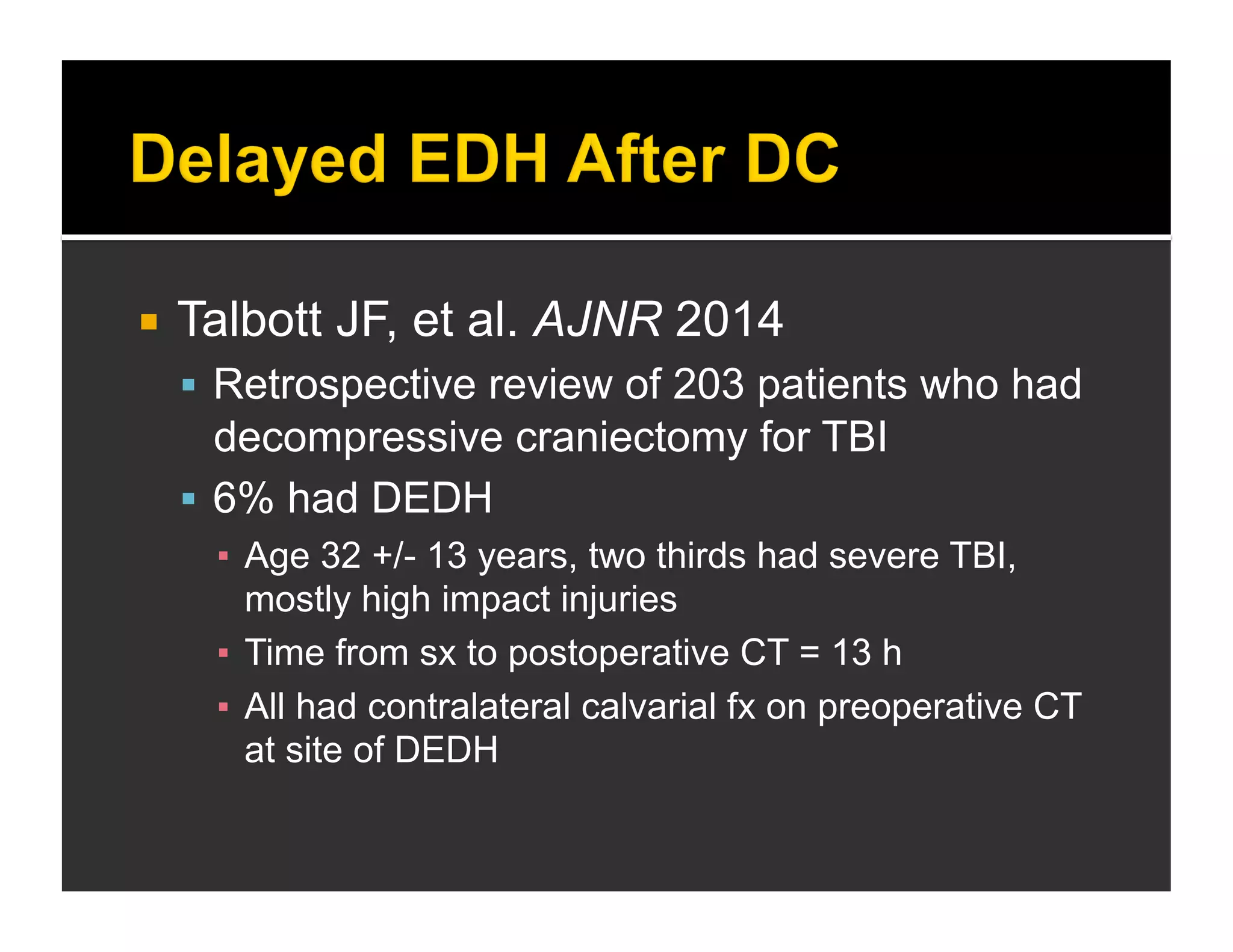
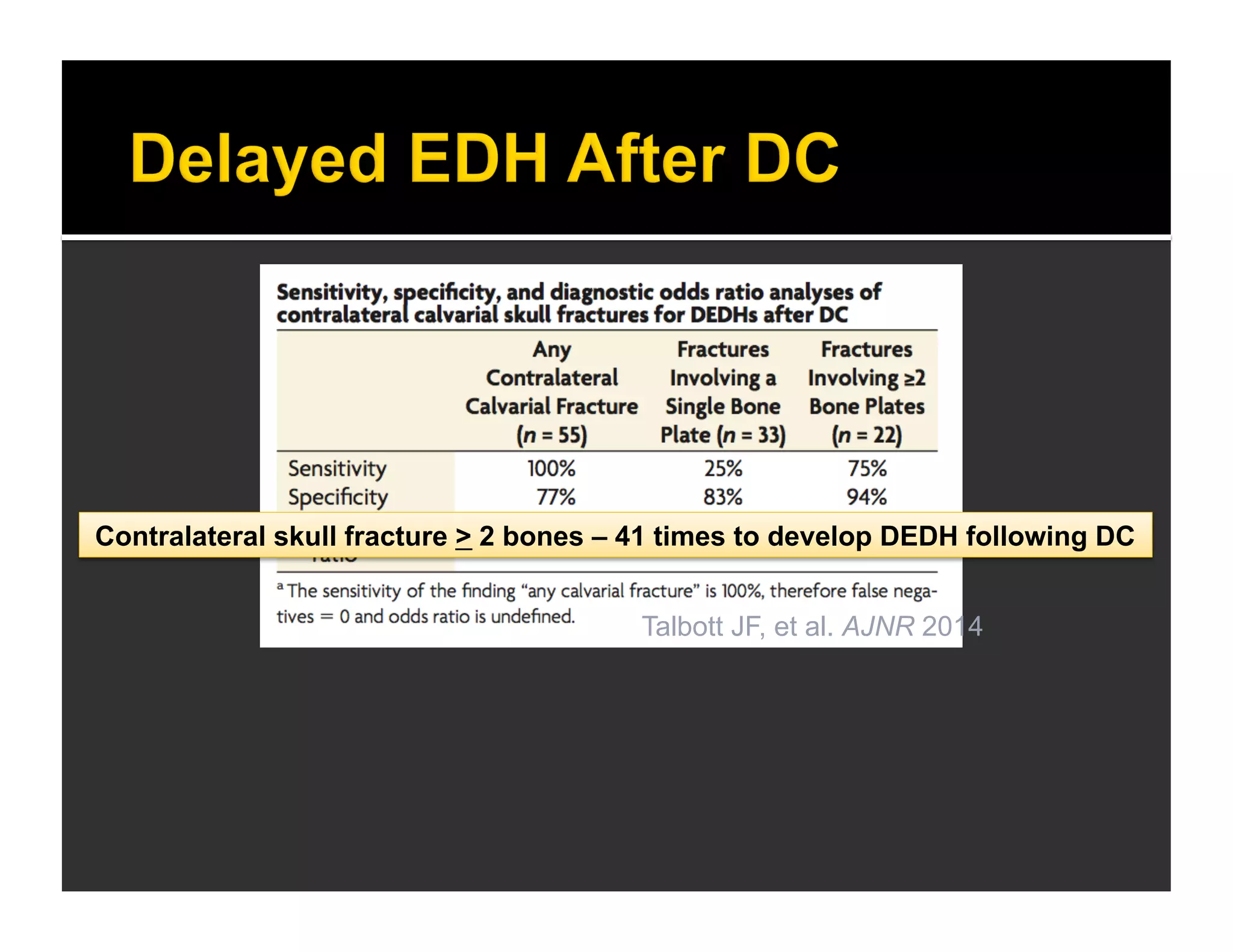
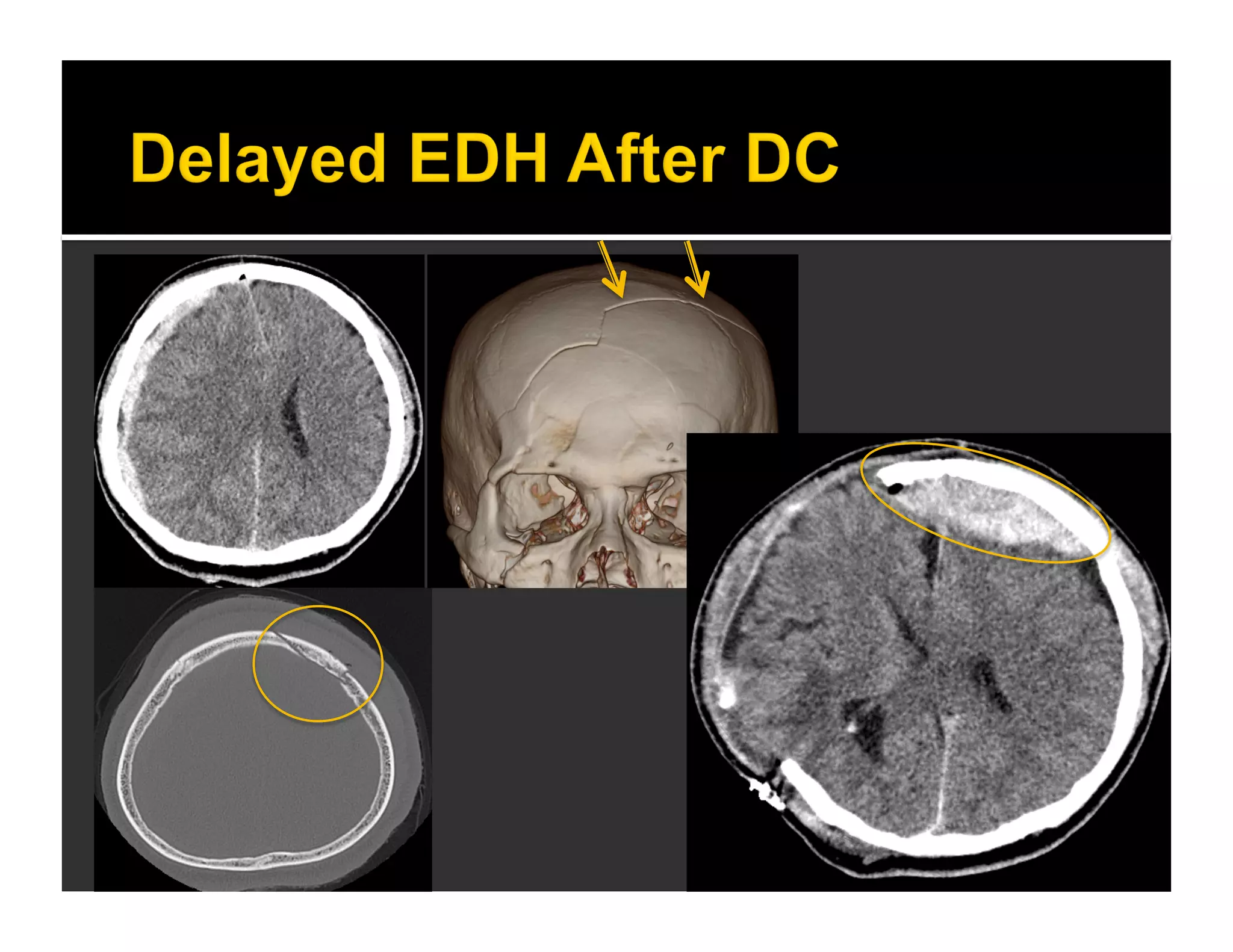
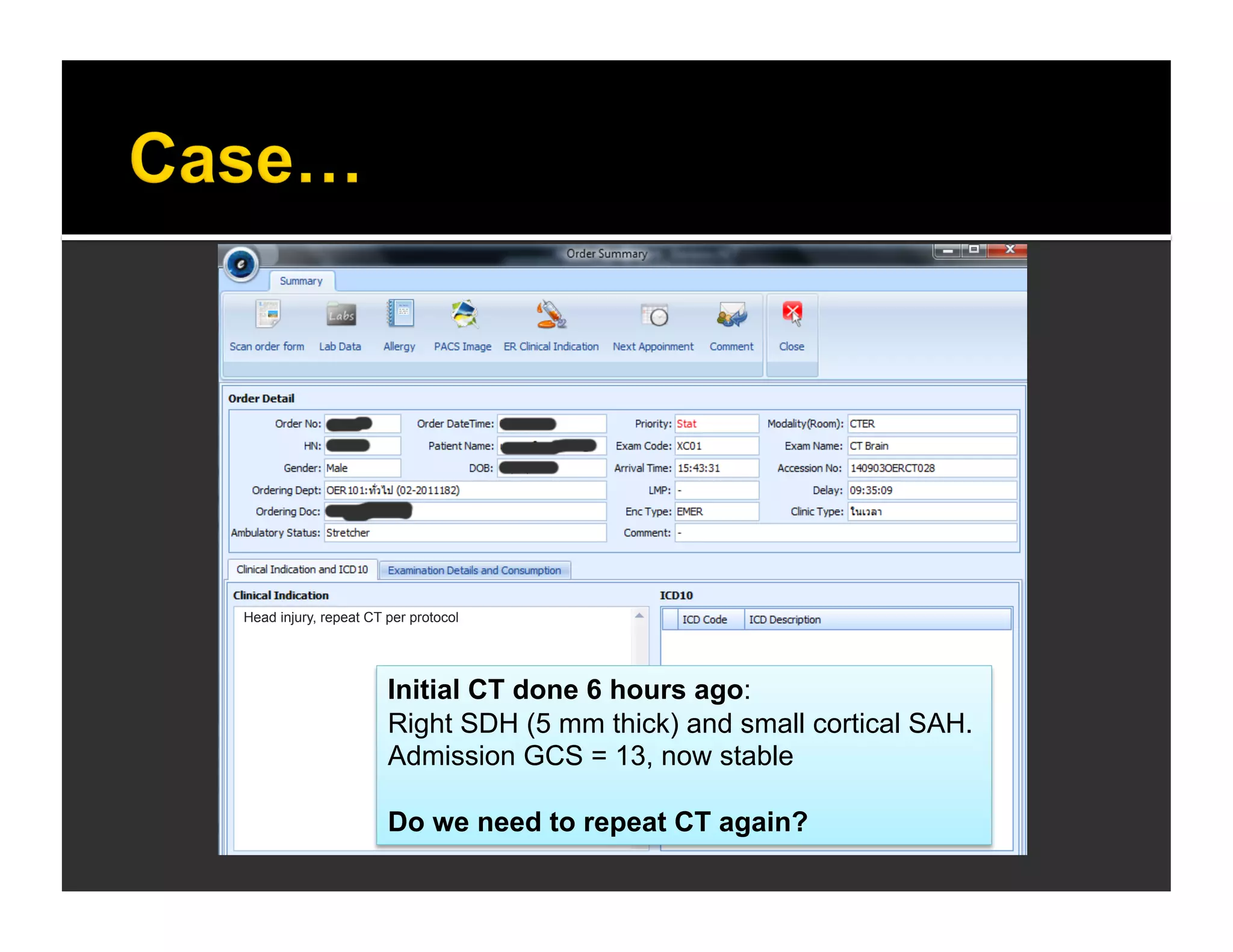
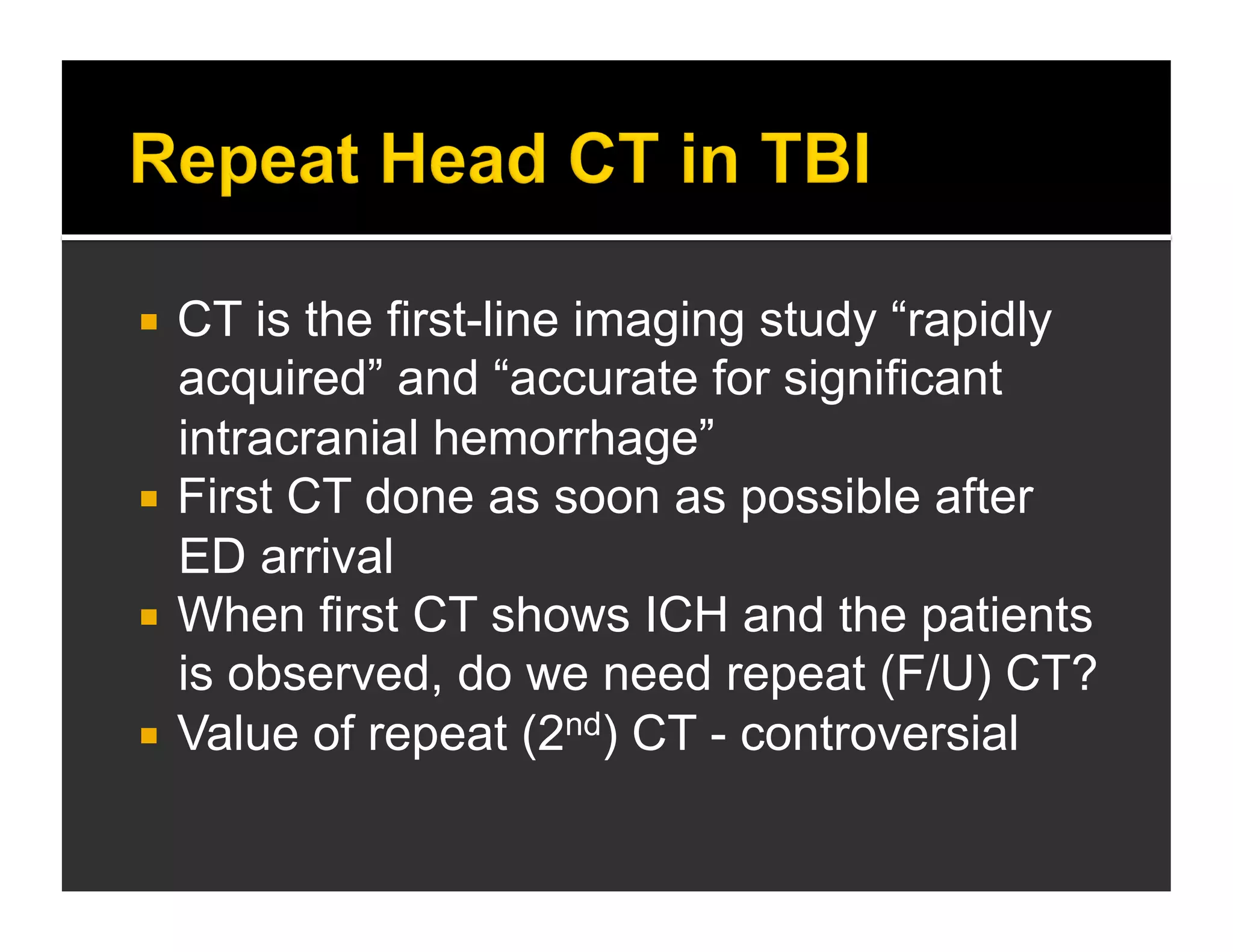
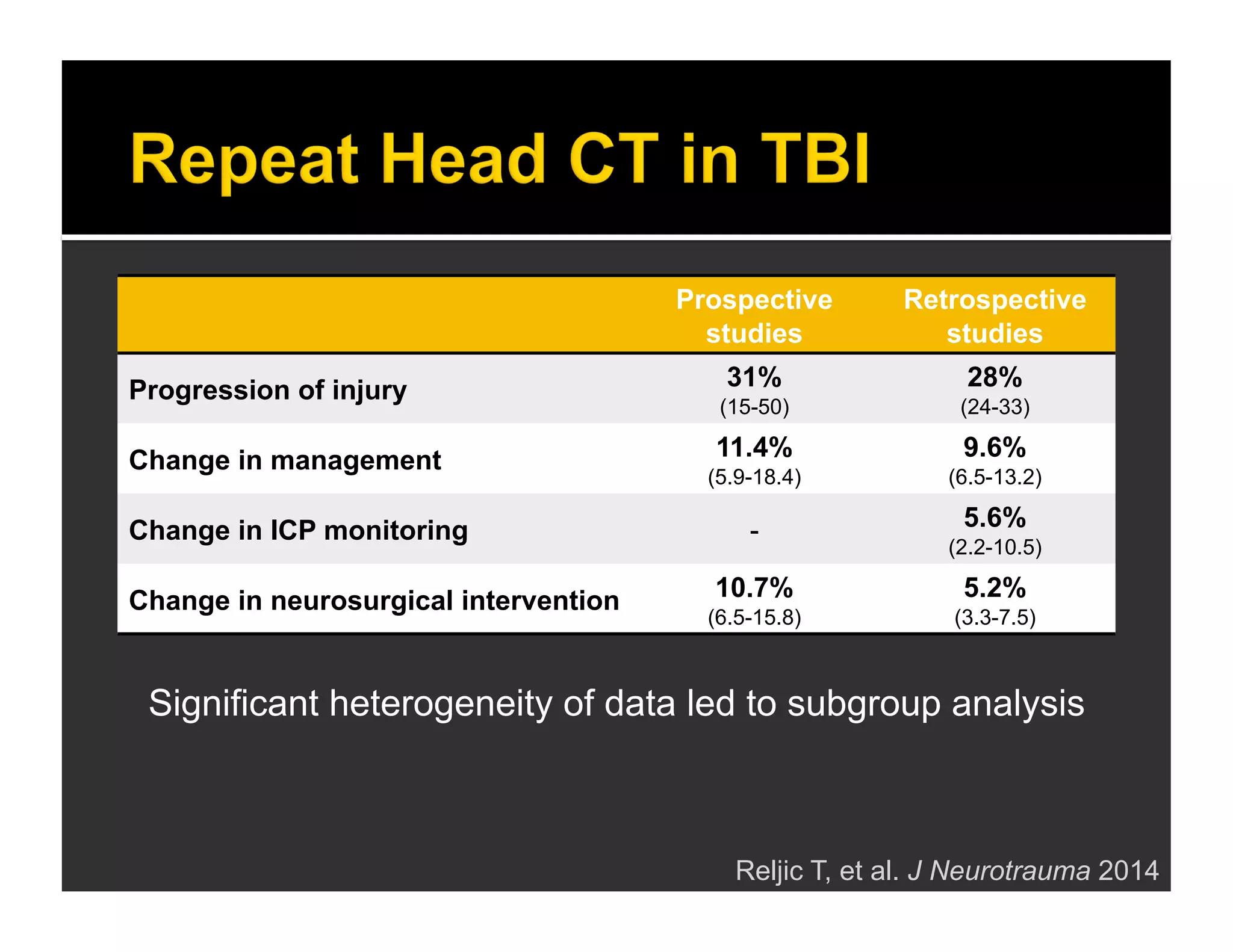
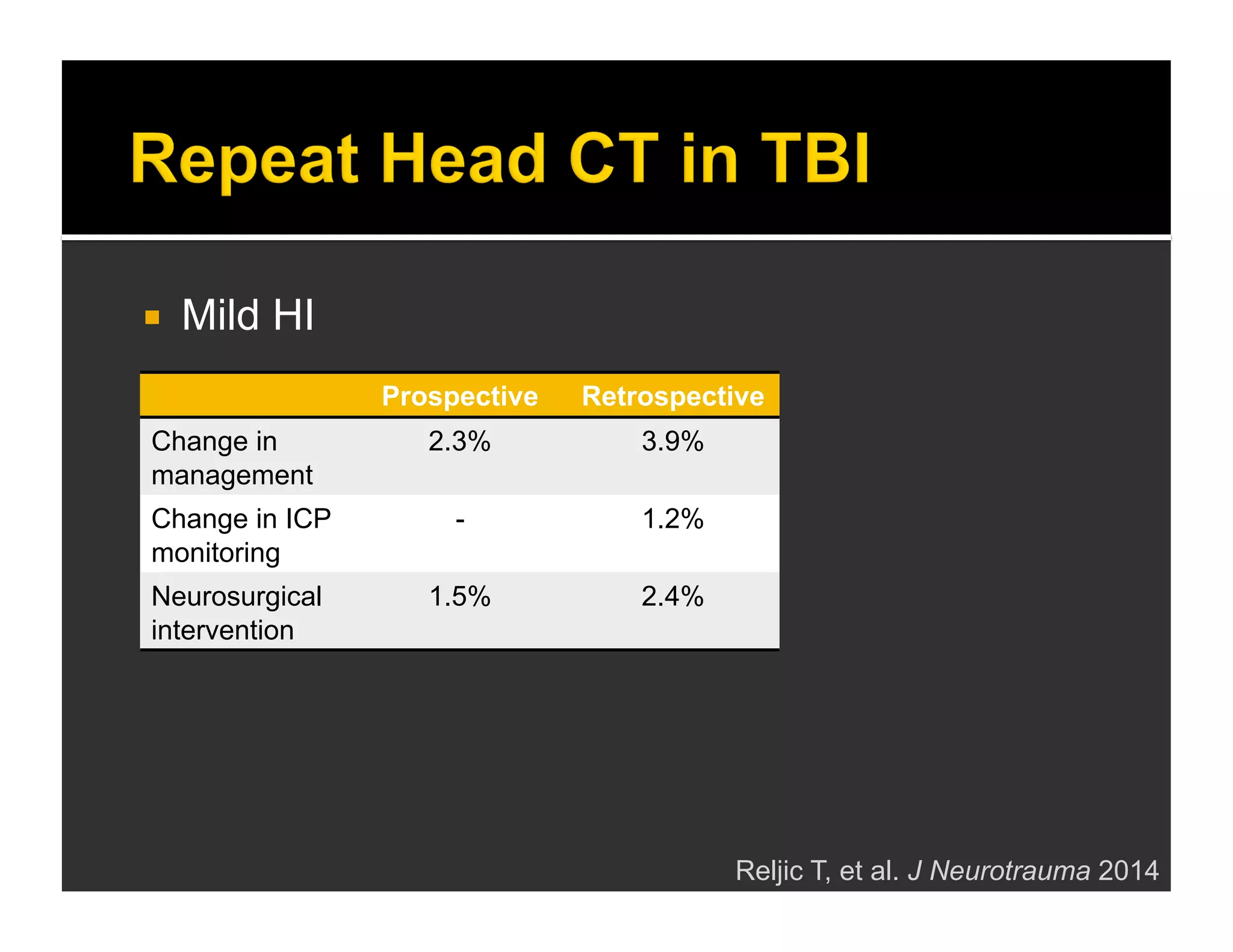
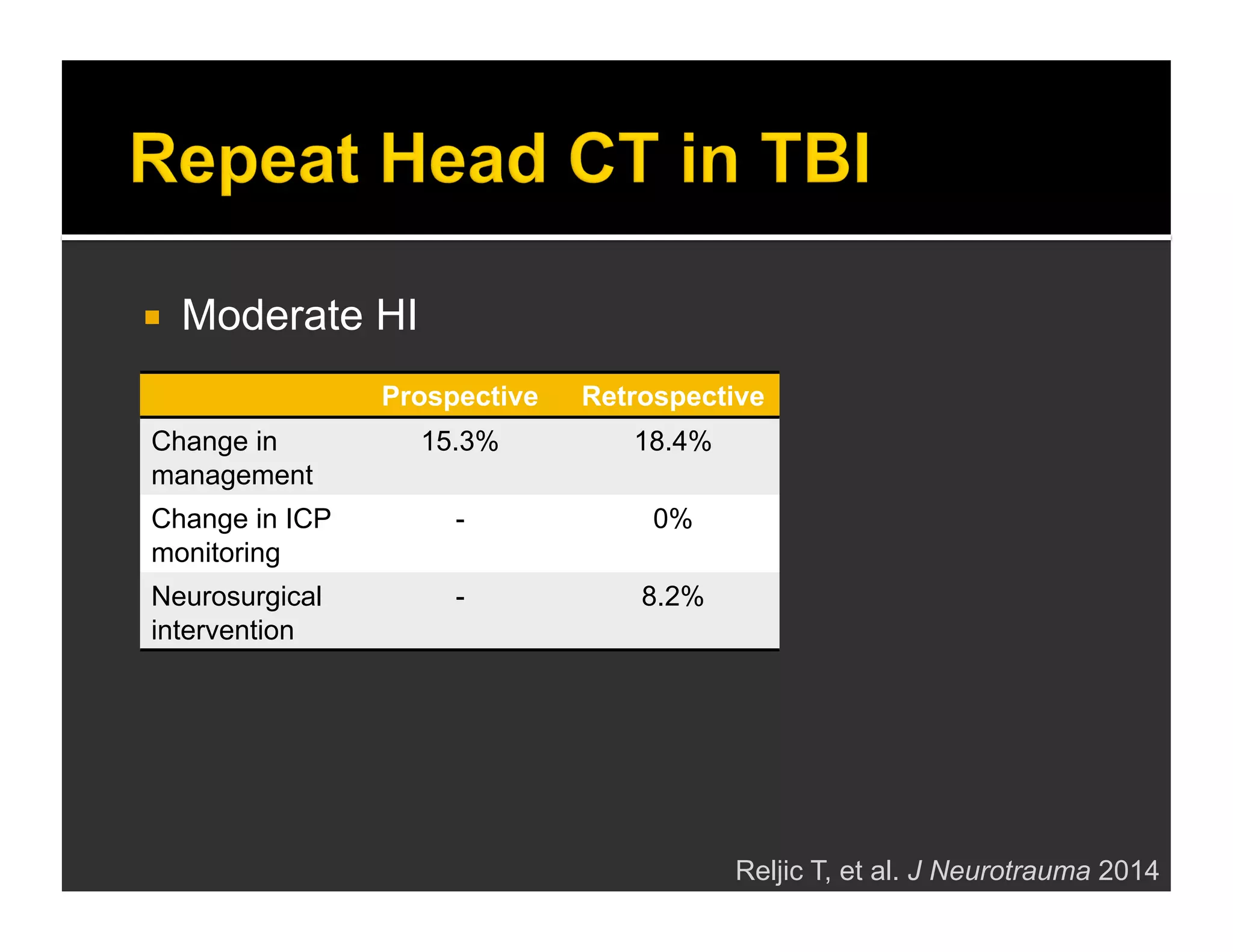
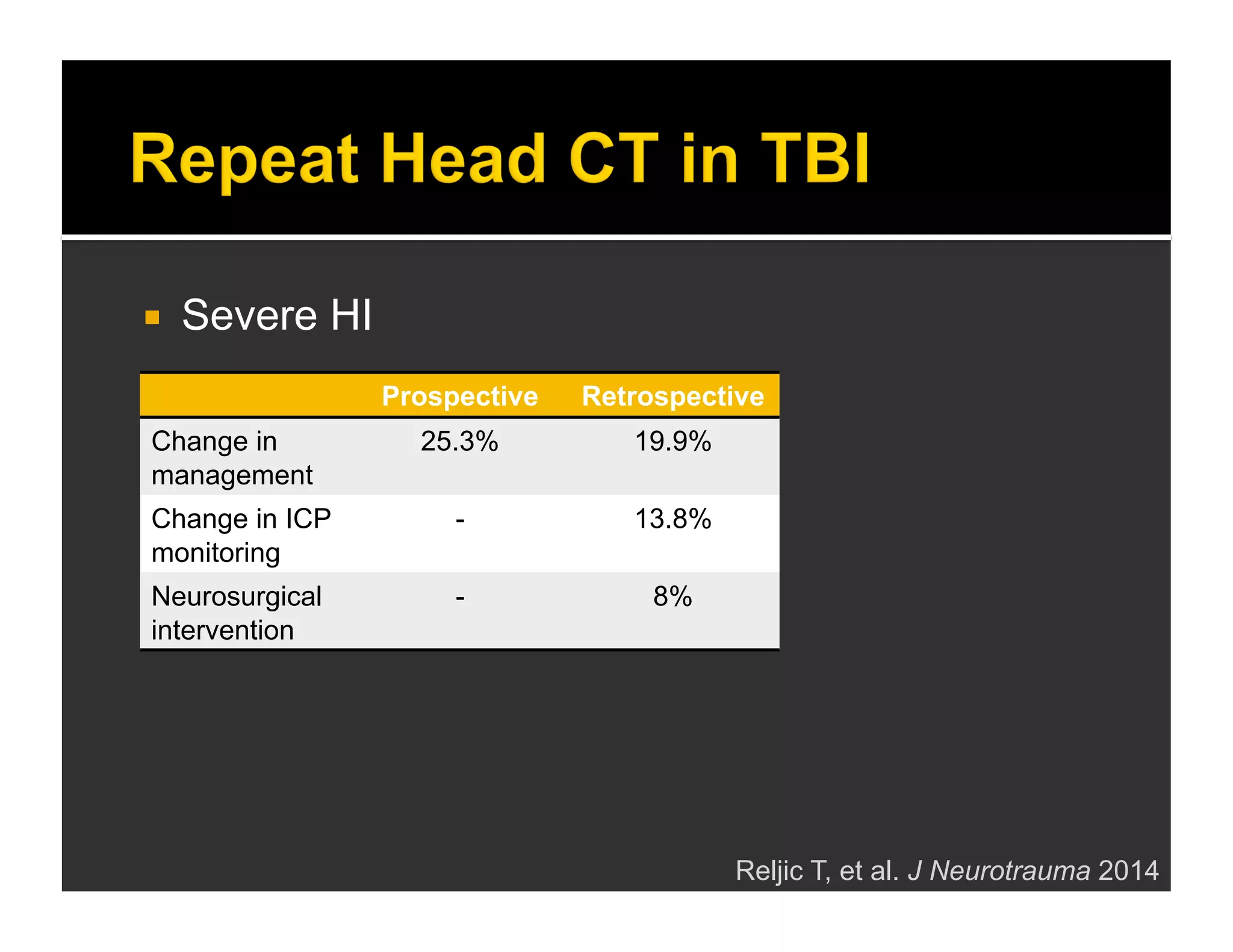
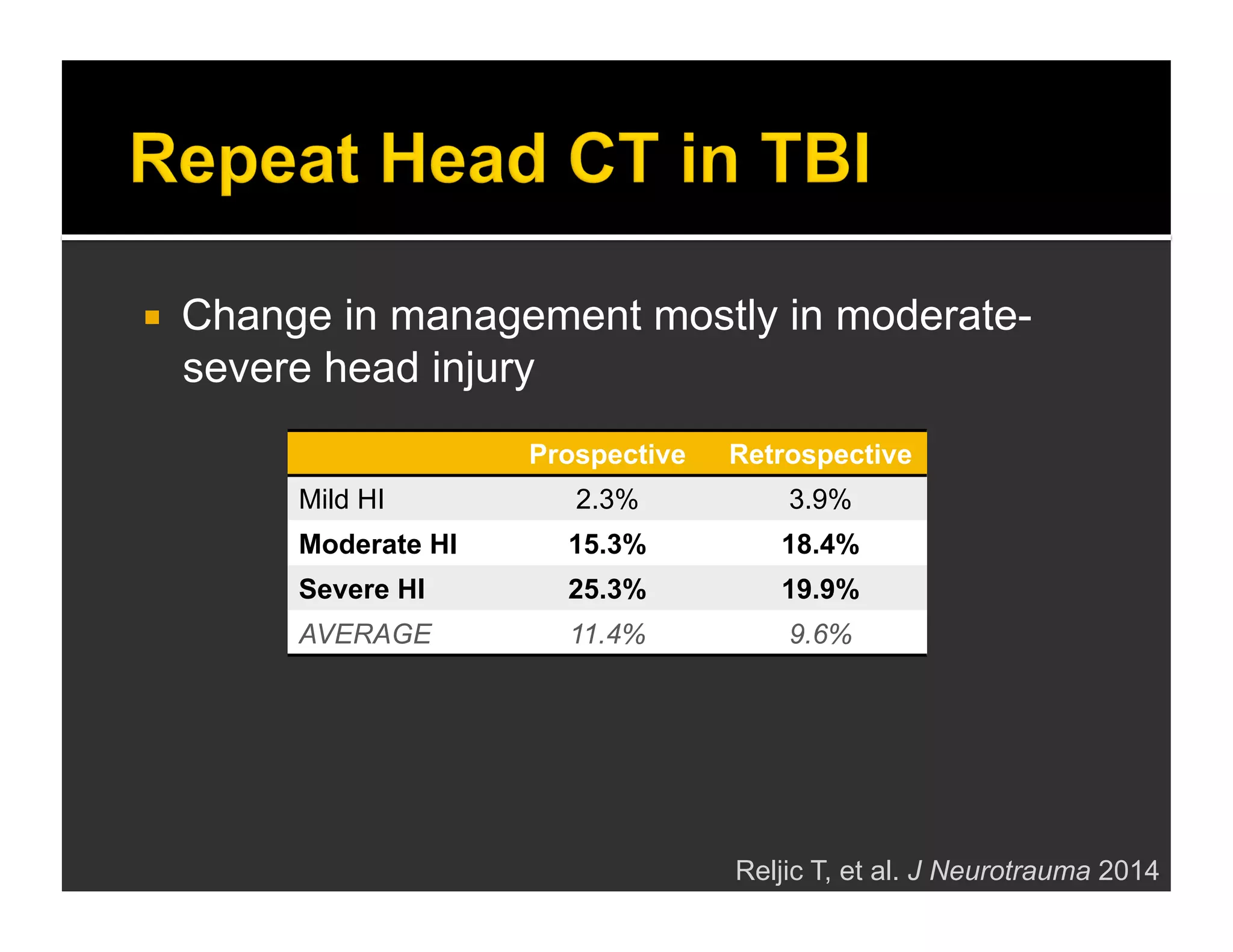
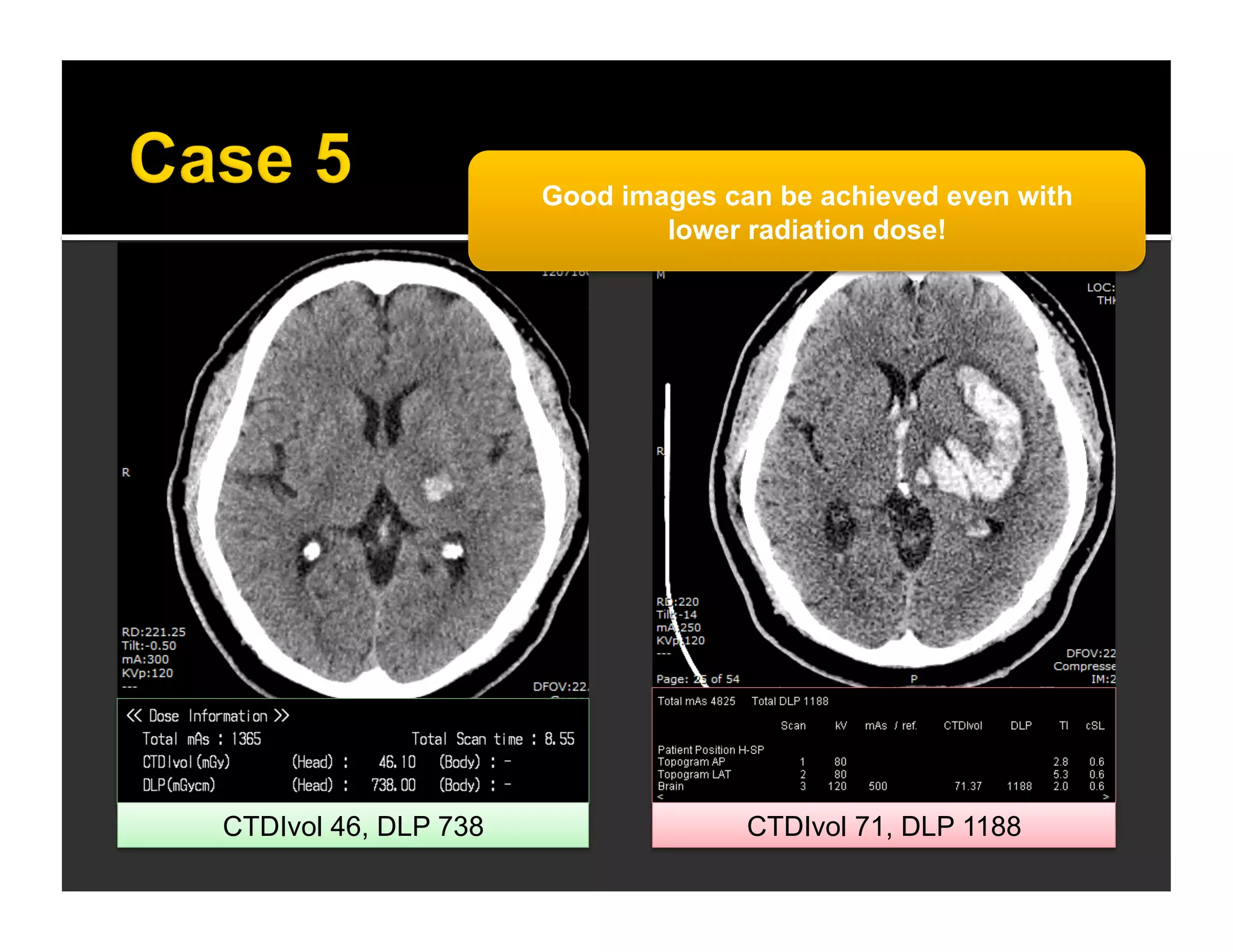
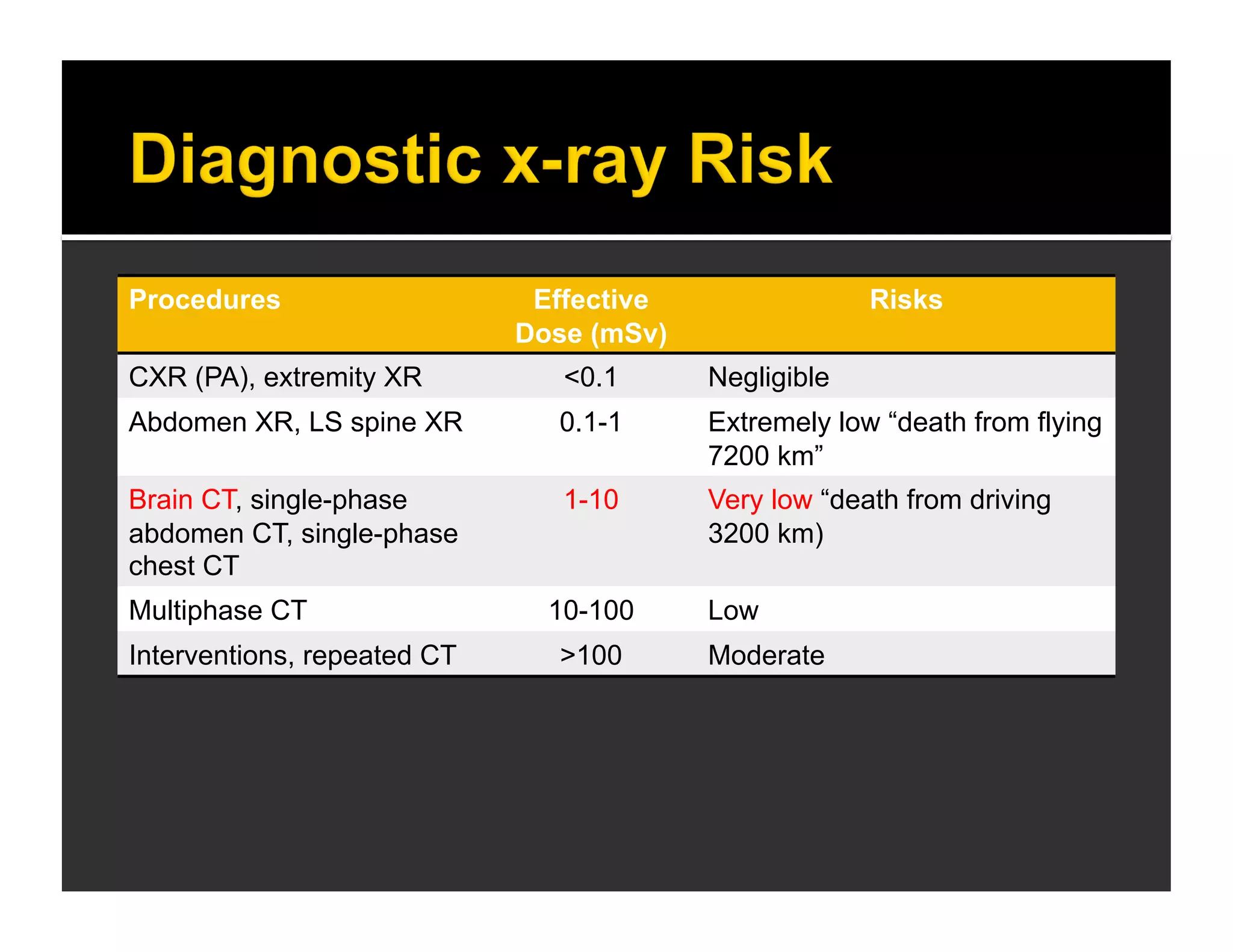
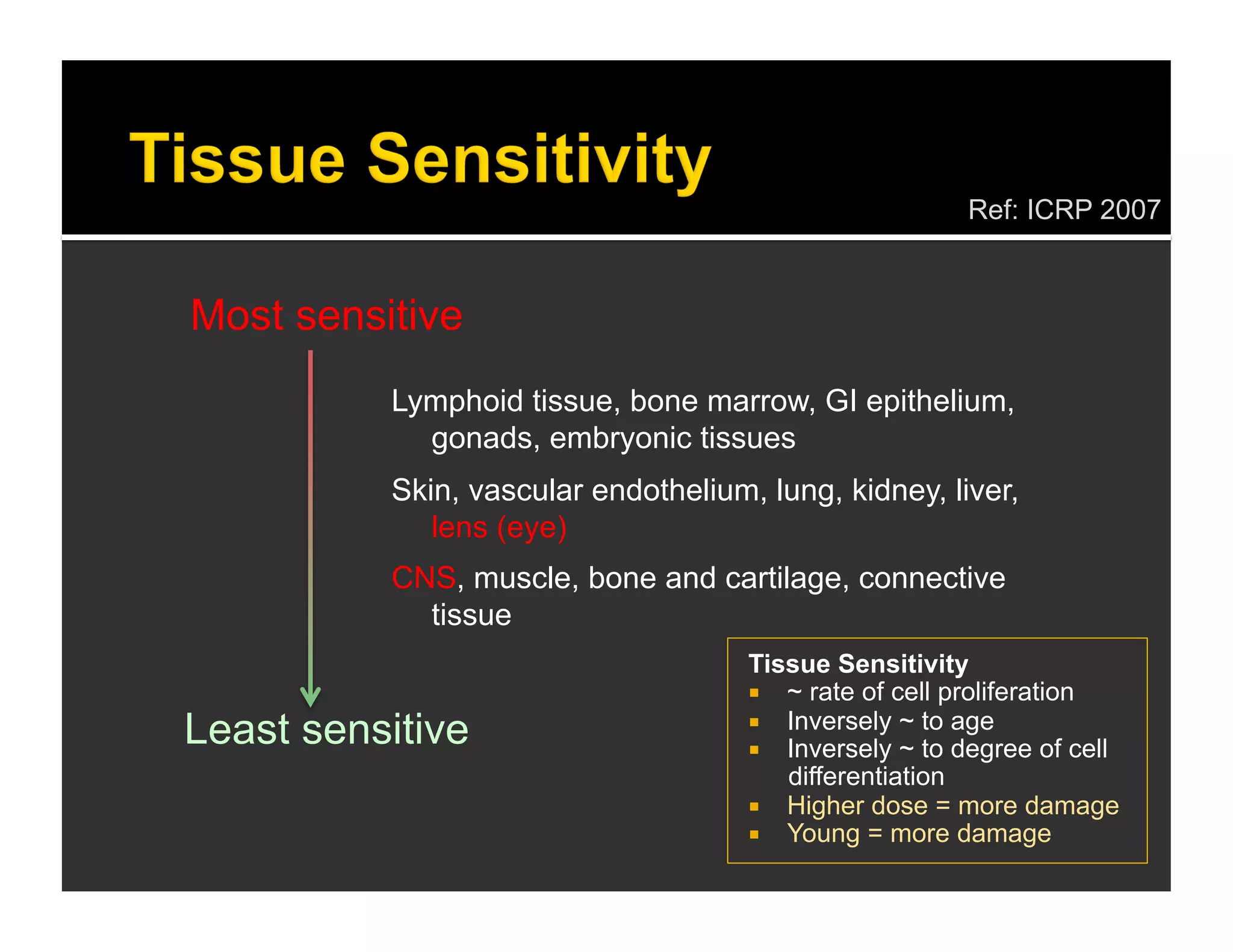
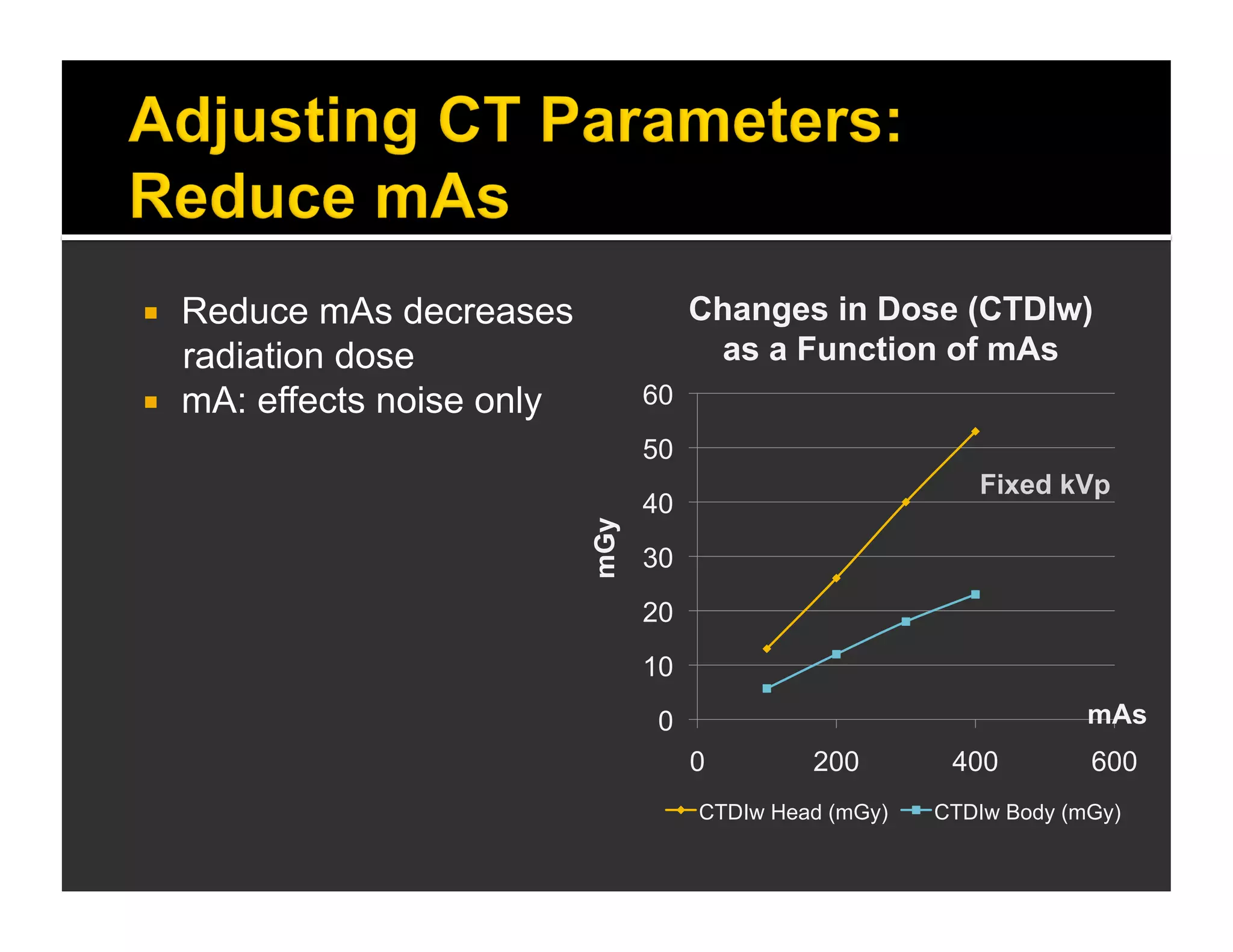
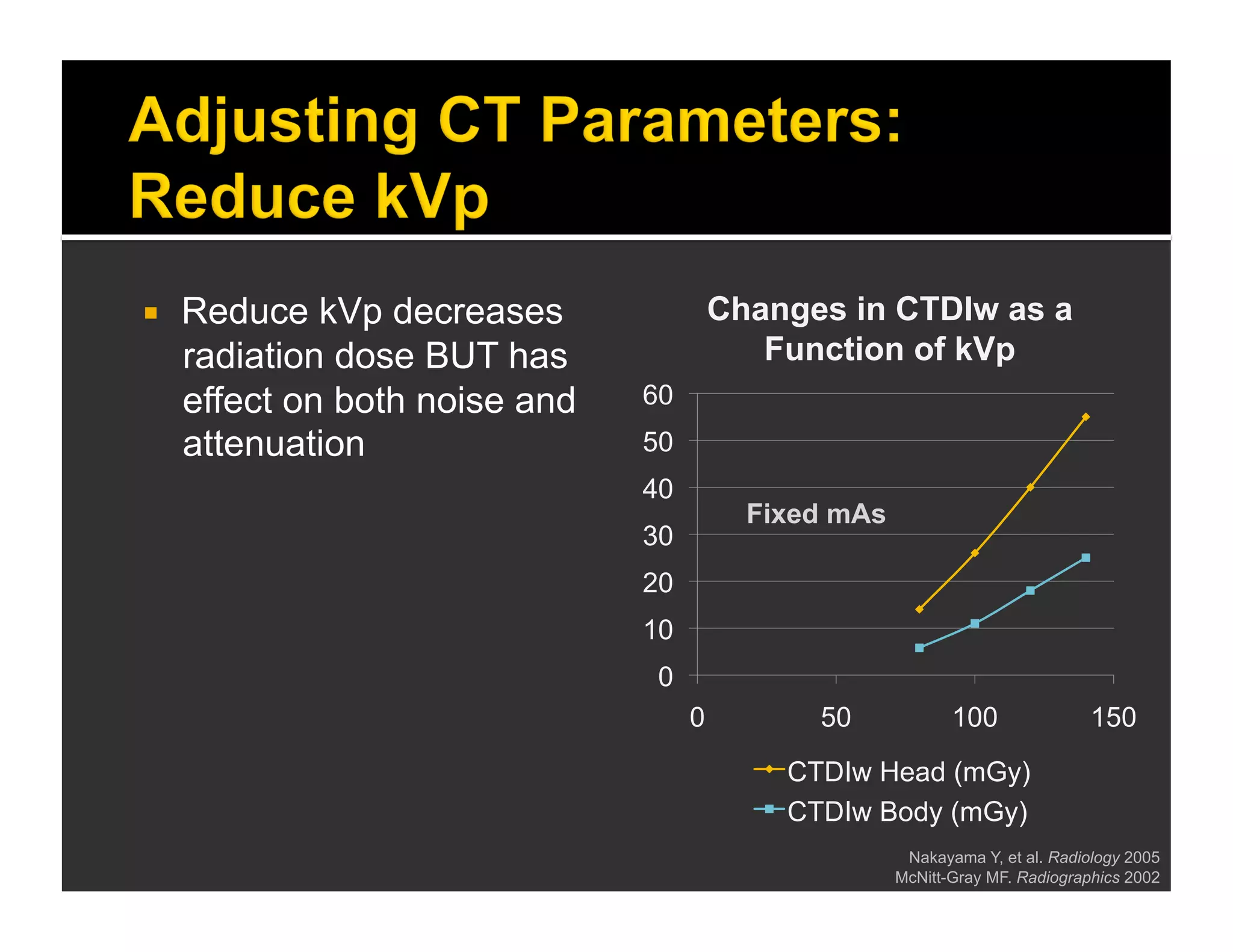
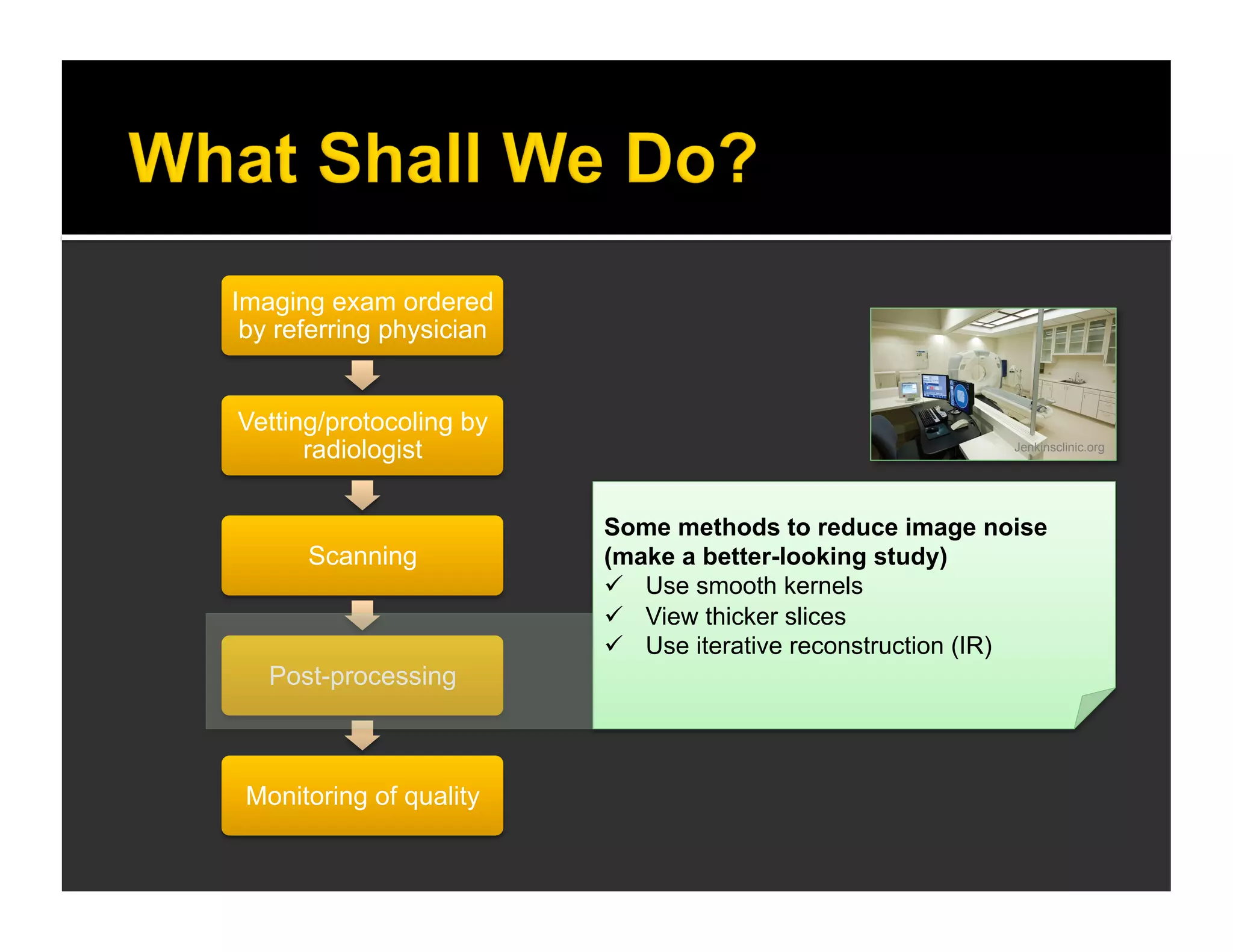
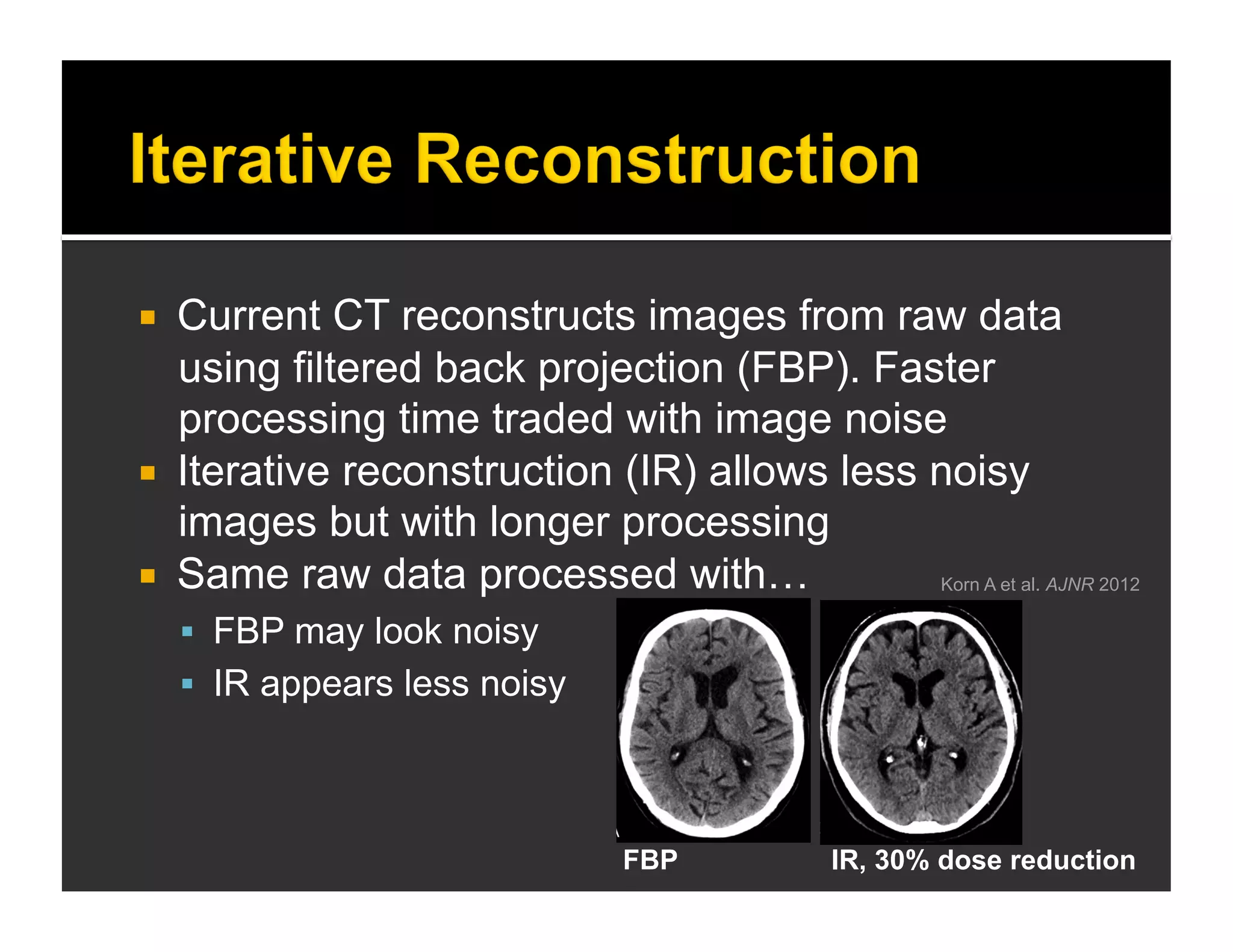
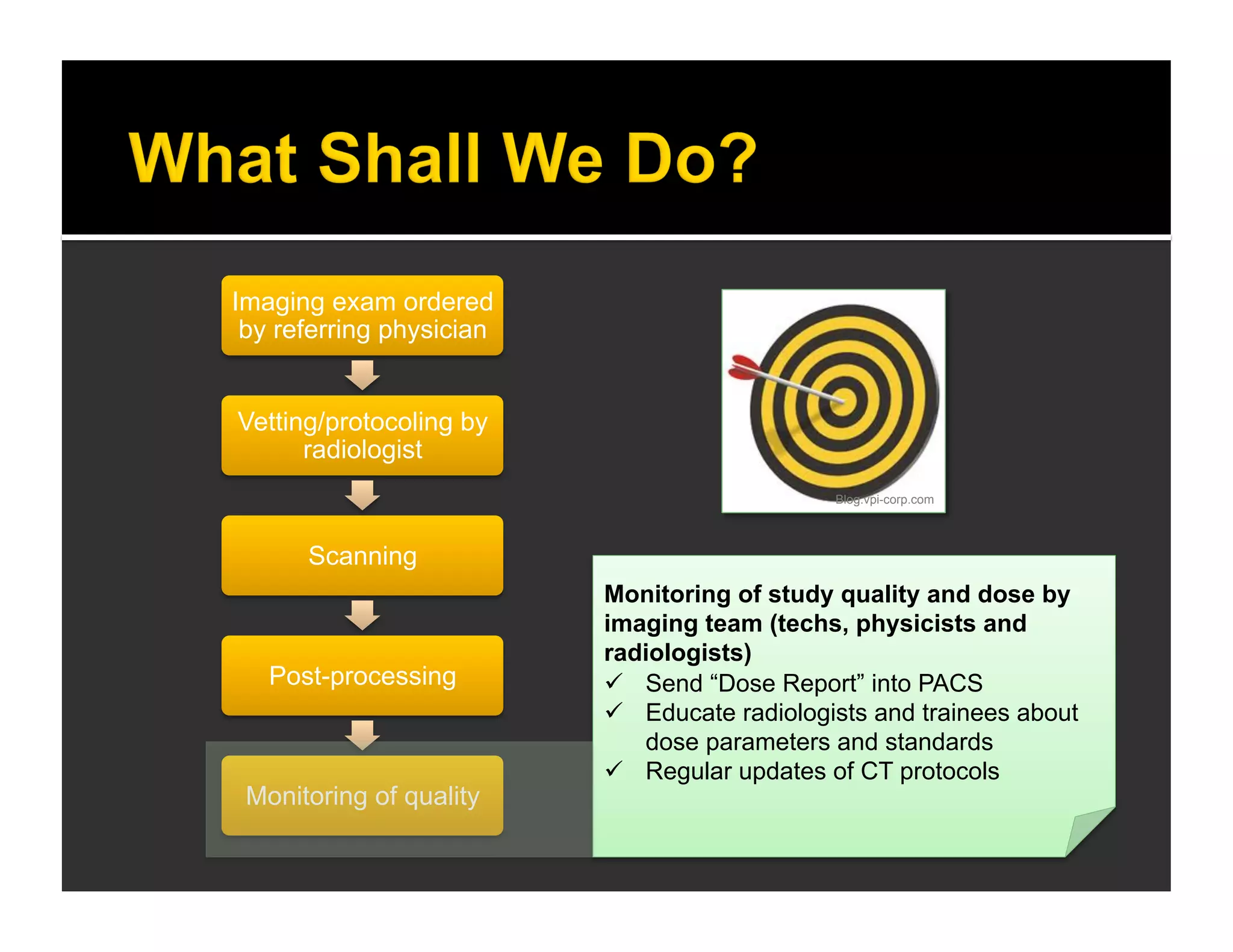
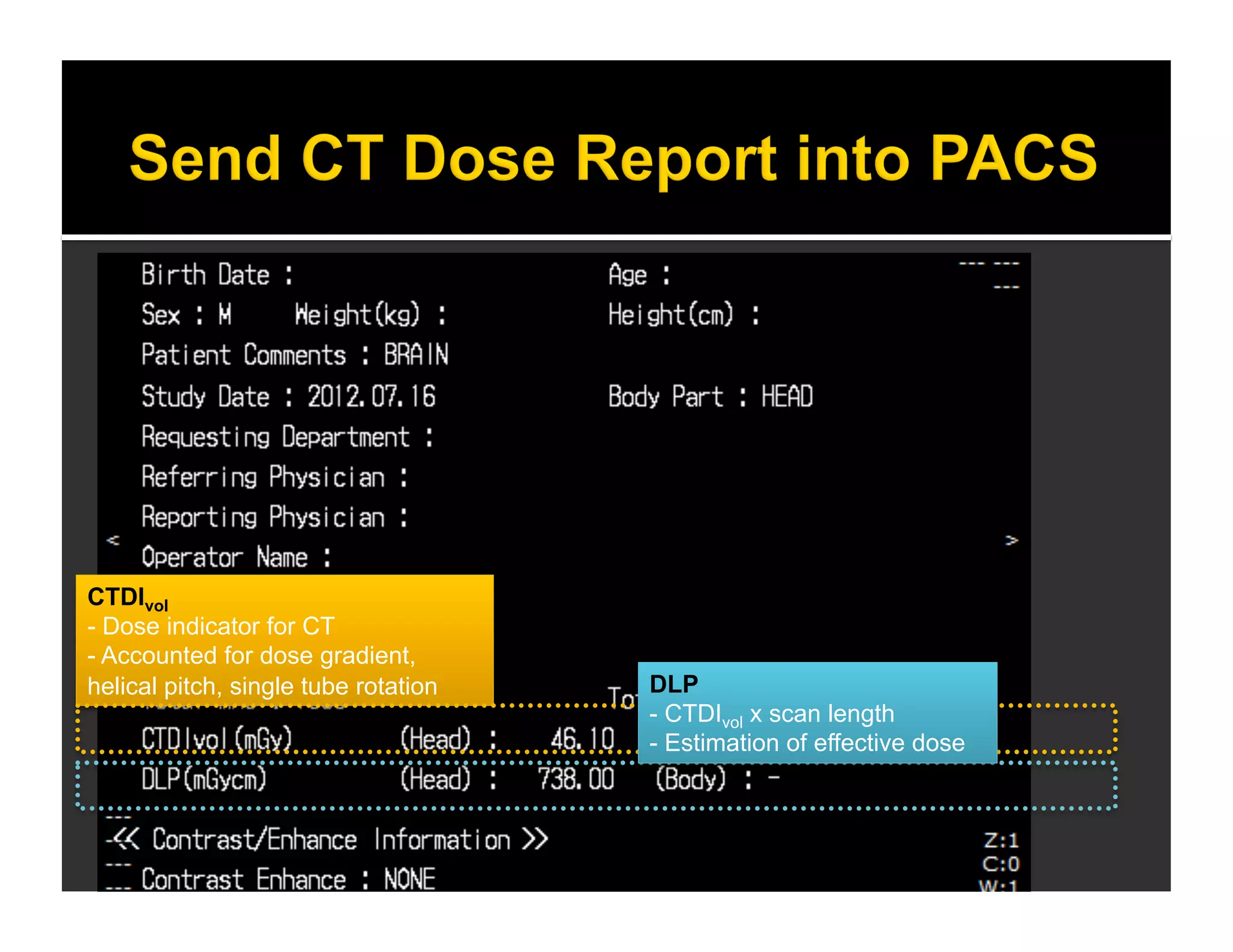
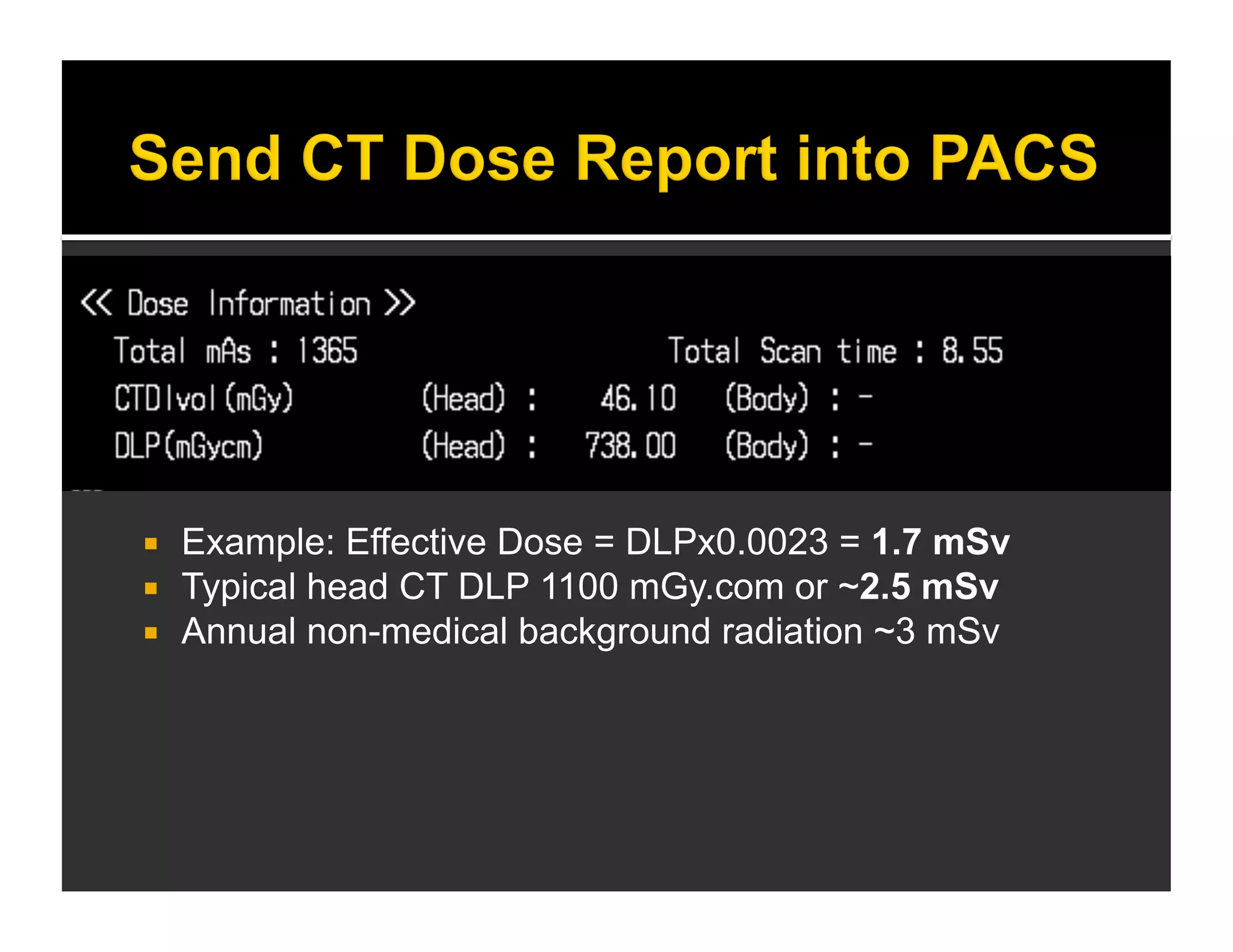
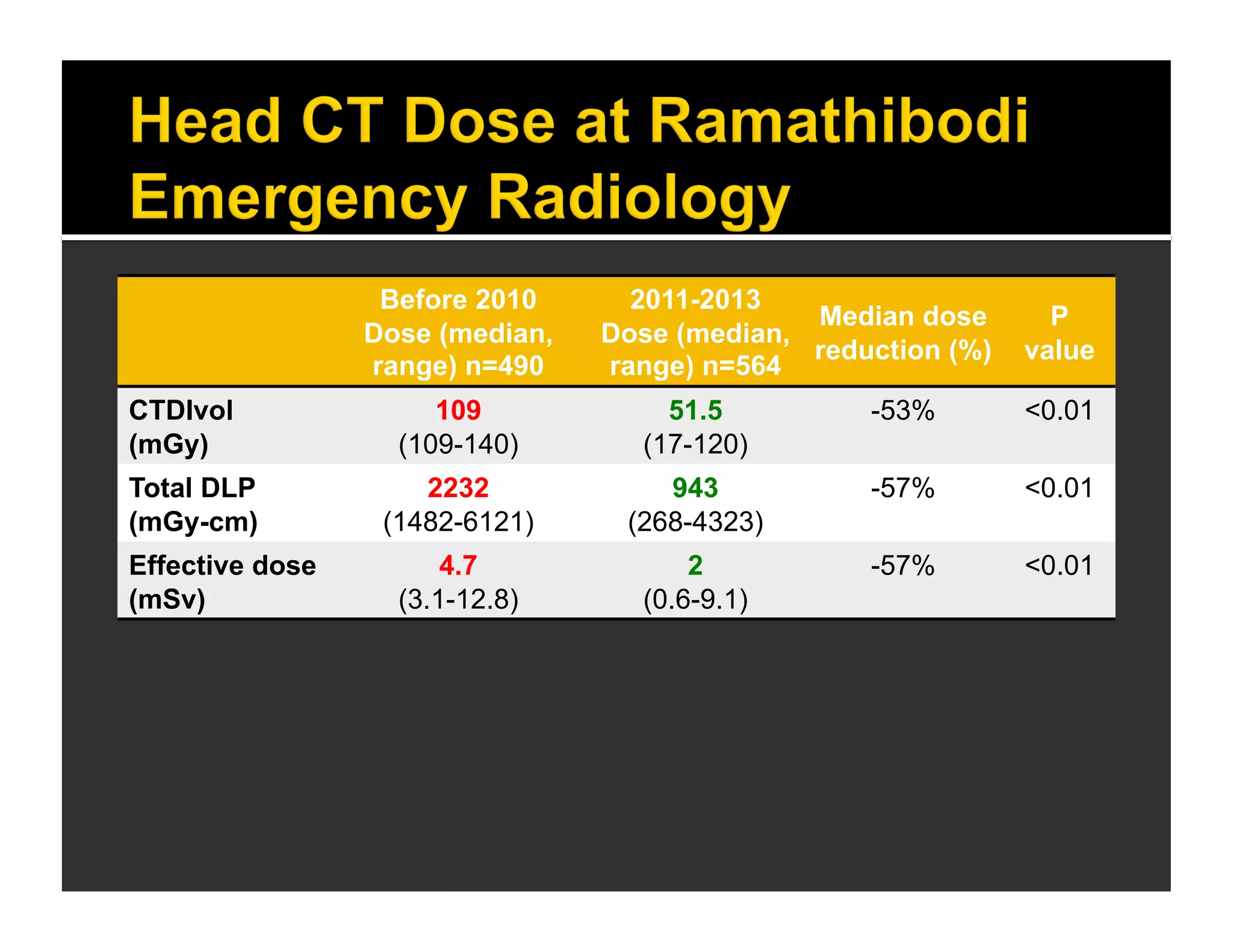
The document discusses the management and diagnosis of head trauma, emphasizing the importance of timely CT imaging in emergency settings. It highlights key factors in prognostic assessment, including specific CT findings and the risks associated with repeat imaging and radiation exposure. Additionally, it covers aspects of treatment strategies such as decompressive craniectomy and approaches to minimizing radiation doses during imaging procedures.