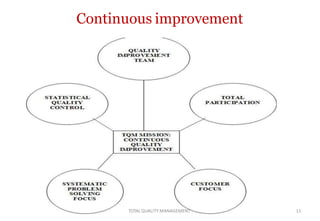
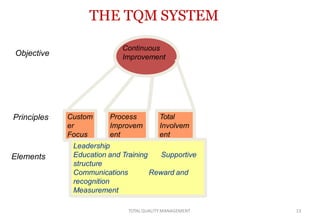
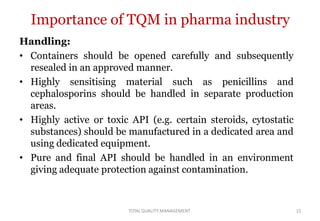
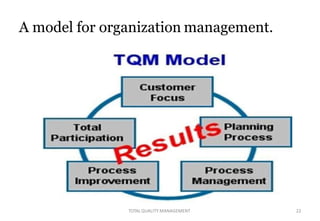
This document discusses Total Quality Management (TQM). It defines TQM as managing the whole organization to achieve excellence. The key concepts of TQM include producing quality work the first time, focusing on customers, having a strategic improvement approach, and encouraging continuous improvement and teamwork. The document outlines the principles, elements, and benefits of TQM, including improved quality, productivity, customer satisfaction, and profitability. It also discusses the importance and applications of TQM in the pharmaceutical industry for handling, storing, packaging, and labeling materials.