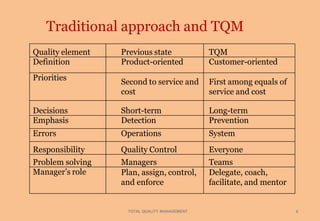
Total quality management (TQM) is a management approach focused on quality that involves all departments and levels of an organization. The key elements of TQM include focusing on customers, employee involvement, and continuous improvement. TQM aims to prevent defects by addressing the underlying systems and processes rather than just detecting defects. Some benefits of TQM include improved quality, increased customer satisfaction, higher employee morale, and lower costs.