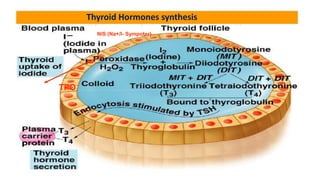
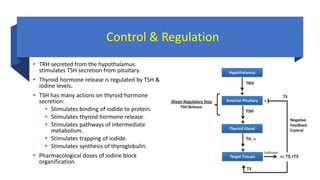
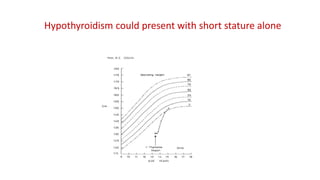
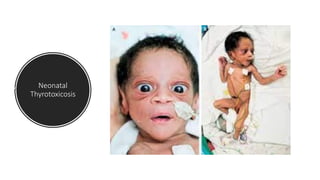
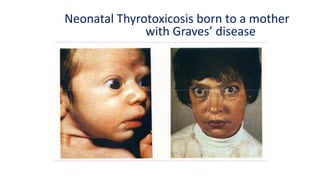
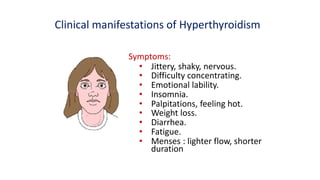
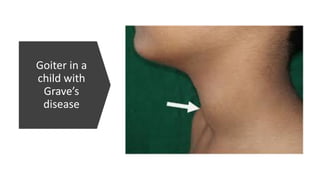
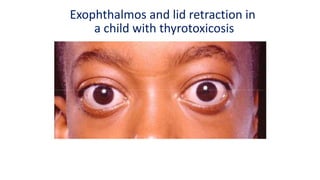
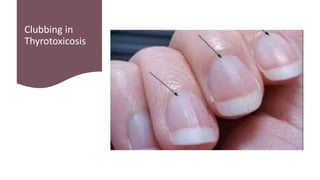
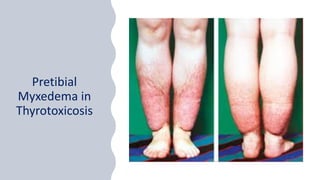
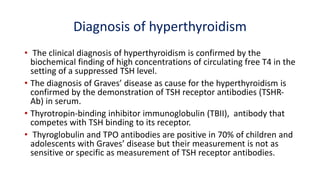
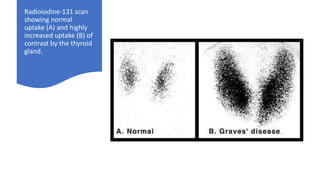
This document discusses thyroid disorders in children from birth to adolescence. It begins with an introduction on the anatomy and physiology of the thyroid gland and thyroid hormone biosynthesis. It then covers congenital hypothyroidism including screening, causes, clinical presentations, investigations and treatment. Transient congenital hypothyroidism and acquired hypothyroidism are also discussed. The document concludes with sections on thyrotoxicosis including investigations and treatment of thyrotoxicosis in children.